Zinc Hydroxide exists as a rare natural mineral in the earth. It is an amphoteric white solid compound that has the ability to dissolve in a solution of strong acid or base. It occurs as three rare earth minerals Wulfingite, ashoverite, and sweetite.
Properties Of Zinc Hydroxide
| Chemical formula | Zn(OH)2 |
| Molecular weight | 99.394 g/mol |
| Density | 3.053 g/cm3 |
| Melting point | 125 degree Celsius. |
Zinc Hydroxide Structural Formula
Here is the typical structural representation of Zinc Hydroxide.
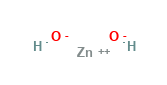
It exists in orthorhombic or tetragonal forms.
In normal conditions, zinc dissociates to form zinc ions along with 2 hydroxide ions from the sodium hydroxide solution to form zinc hydroxide.
Zn2++ 2OH- → Zn(OH)2
When Zinc hydroxide reacts with an excess of sodium hydroxide, it precipitates to form Zn (OH)2 and later dissolves into zincate ion.
Zn (OH)2 + 2OH– → Zn(OH)42-
For more understanding of the concept refer to BYJU’S!!
Comments