Zinc Iodide is a chemical compound made of zinc and iodine having a molecular formula ZnI2. It is a white solid that readily absorbs water from the atmosphere. It can be manufactured directly by the reaction of zinc and iodine while refluxing ether. In this short piece of article, let us learn more about the zinc iodide formula, its properties, chemical structure and uses.
Zinc Iodide Properties
| Properties of Zinc Iodide | |
| Name | Zinc Iodide |
| Appearance | White powder |
| Chemical Formula | ZnI2 |
| Melting Point | 446 °C |
| Boiling Point | 1,150 °C |
| Density | 4.74 g/cm³ |
| Molar Mass | 319.22 g/mol |
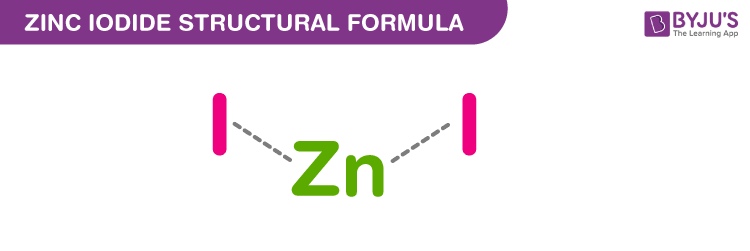
Zinc Iodide Chemical Structure
Zinc Iodide Uses
- Used as a stain in electron microscopy
- Used as an X-ray opaque penetrant in industrial radiography
- Used as a catalyst in the conversion of methanol to triptane
To learn more about such chemistry topics register to BYJU’S now!
Comments