Aluminum Bromide formula, also known as Cupric chloride formula or Aluminium tribromide formula is explained in this article. The most common form of aluminium bromide is Aluminium tribromide. It is a colourless, hygroscopic solid, and sublimable. The molecular or chemical formula of Aluminum Bromide is AlBr3.
In its anhydrous form, it appears as a white to a yellowish-red compound which is lumpy solid and has a pungent odour. In its aqueous form, it appears as a liquid. It is very corrosive to eyes, mucous membranes, and skin. It is widely used to make various chemicals. It acts as a sol in many organic solvents such as benzene, toluene, simple hydrocarbons, nitrobenzene, and xylene.
Aluminum Bromide Formula Structure
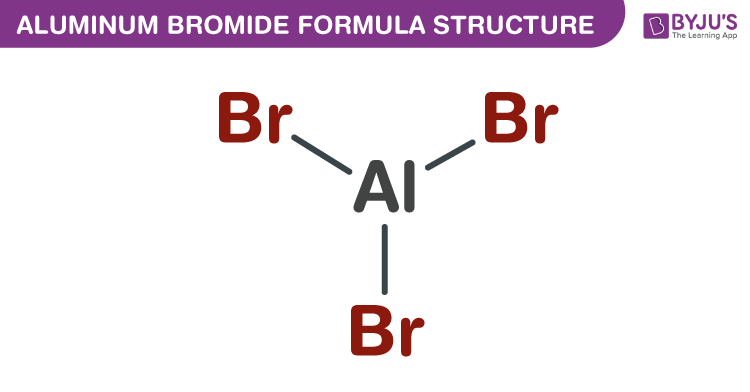
Properties Of Aluminum Bromide
| Chemical formula | AlBr3 |
| Molecular weight | 266.694 g/mol (anhydrous)
374.785 g/mol (hexahydrate) |
| Density | 3.2 g/cm3 (anhydrous)
2.54 g/cm3 (hexahydrate) |
| Boiling point | 255 °C (anhydrous) |
| Melting point | 97.5 °C (anhydrous)
93 °C (hexahydrate) |
Safety Measures
- Aluminum Tri Bromide is a highly reactive material.
To learn more about Aluminum Bromide formula from the expert faculties at BYJU’S, register now!
Comments