Aluminium fluoride is a colourless chemical compound with a molecular formula AlF3. It is mainly used in the production of aluminium. It occurs naturally as oskarssonite and rosenbergite. It can be manufactured synthetically too. The majority of aluminium fluoride is produced by treating hydrogen fluoride with alumina or aluminium oxide at 700 °C. In this short piece of article, learn more about aluminium fluoride formula, its chemical structure, properties and uses.
Aluminium Fluoride Properties
| Properties of Aluminium Flouride | |
| Name | Aluminium Fluoride |
| Appearance | Hygroscopic white or colourless solid |
| Chemical Formula | AlF3 |
| Melting Point | 1,291 °C (anhydrous) |
| Molar Mass | 83.977 g/mol (anhydrous)
101.992 g/mol (monohydrate) 138.023 g/mol (trihydrate) |
| Density | 3.10 g/cm3 (anhydrous)
2.17 g/cm3 (monohydrate) 3.10 g/cm3 (trihydrate) |
| Solubility in Water | Soluble in water |
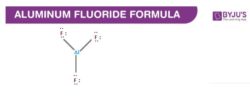
Aluminium Fluoride Structure
Aluminium Fluoride Uses
- Used as an additive for the production of aluminium by electrolysis
- Used in the fermentation process in beer and wine factories
- Used in the production of low index optical thin film
- Used as a catalyst in the organic compound synthesis process
To learn more about such chemistry topics register to BYJU’S now!
Comments