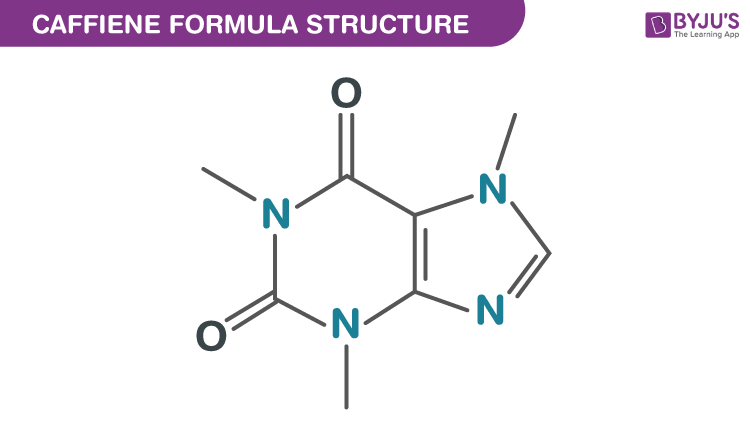
Caffeine, also known by the name IUPAC name 1, 3, 7-Trimethylpurine-2,6-dione, has a chemical formula C8H10N4O2. Caffeine is found in common food and beverages that we consume every day, such as coffee and tea. Coffee and tea leaves contain a maximum of 5 % of the caffeine in them. In chemical laboratories, caffeine is prepared by the reaction between dimethylurea and malonic acid. In this short piece of article, let us learn more about the caffeine chemical formula, its chemical and physical properties along with its chemical structure.
Physical Properties of Caffeine
| Caffeine Physical Properties | |
| Name | Caffeine |
| Also Known as | Trimethylxanthine, mateine, theine, guaranine, methyltheobromine |
| Appearance | Odourless, white needles or powder |
| Molecular Formula | C8H10N4O2 |
| Melting Point of Caffeine | 235 °C |
| Boiling Point of Caffeine | 178 °C |
| Density | 1.23 g/cm³ |
| Molar Mass | 194.19 g/mol |
| Solubility in Water | Slightly Soluble |
Chemical Structure of Caffeine
Caffeine is an alkaloid and is formed by pyrimidinedione and imidazole rings which are fused together. It should be noted that pyrimidinedione is a 6-member ring having 2 nitrogen atoms while an imidazole ring is a 5-member ring having 2 nitrogen atoms.
Caffeine Uses
- Commonly used stimulants in athletes
- Caffeine creams are applied to the skin to reduce redness and itching in dermatitis.
- Caffeine is used as an ingredient in energy drinks, soft drinks and other beverages.
To learn more about such chemistry topics register to BYJU’S now!
Comments