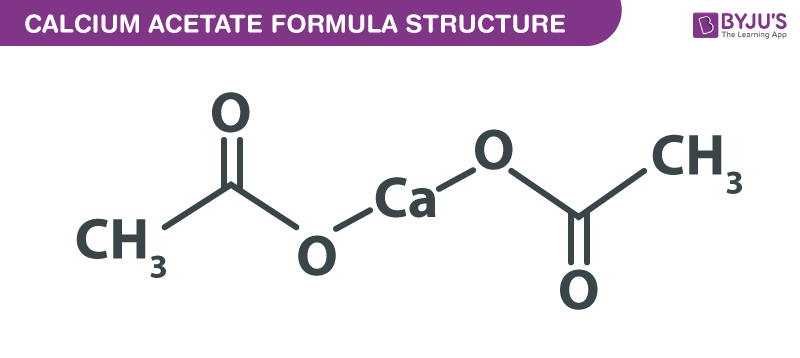
Calcium Acetate formula, also known as Calcium Diacetate formula or Acetate Of Lime formula is explained in this article. This chemical compound is the calcium salt of acetic acid. It consists of four carbon atoms, six hydrogen atoms, one calcium atom, and four oxygen atoms. The chemical or molecular formula of Calcium Acetate is C4H6CaO4.
It is colourless to white crystalline solid which has a slight odour of acetic acid. It dissolves in water and alcohol and is insoluble in benzene and acetone. In its anhydrous form is strongly hygroscopic and therefore occurs as monohydrate in its common form. Calcium acetate can be obtained by soaking calcium carbonate or hydrated lime in vinegar. It is widely used in food products as a food additive, buffer, sequestrant, and as a stabilizer.
Calcium Acetate Formula Structure
Properties Of Calcium Acetate Formula
| Chemical formula | C4H6CaO4 |
| Molecular weight | 158.166 g/mol |
| Density | 1.509 g/cm3 |
| Refractive index | 1.55 |
| Melting point | 160°C |
It is used, commonly as a hydrate, in the treatment of hyperphosphatemia in patients with kidney disease.
To learn more about Calcium Acetate formula from the expert faculties at BYJU’S, register now!
Comments