What is Acetaldehyde (CH3CHO)?
C2H4O is an organic chemical compound with the chemical name Acetaldehyde.
Acetaldehyde is also called MeCHO. It is miscible with napthalene, gasoline, xylene, ether, turpentine, alcohol and benzene. It has no colour and is a flammable liquid. It has a suffocating smell. It is non-corrosive to many metals but when It has a narcotic action and can cause mucous irritation.
Table of Contents
- Acetaldehyde Structure
- Acetaldehyde Formula
- Properties of Acetaldehyde
- Preparations of Acetaldehyde
- Chemical Properties of Acetaldehyde
- Acetaldehyde (C2H4O) Uses
- Acetaldehyde Toxicity
- Frequently Asked Questions – FAQs
Acetaldehyde is widely used in the manufacturing of perfumes, drugs, acetic acid, flavouring agent, dyes, etc. When acetaldehyde is applied externally for prolonged periods it is toxic. Acetaldehyde was first observed in the year 1774 by the Swedish pharmacist/chemist Carl Wilhelm Scheele.
Acetaldehyde Structure (C2H4O Structure)
Acetaldehyde Formula
The formula for Acetaldehyde CH3CHO or C2H4O.
Properties of Acetaldehyde – C2H4O
| C2H4O | Acetaldehyde |
| Molecular Weight/ Molar Mass | 44.05 g/mol |
| Density | 0.784 g/cm3 |
| Boiling Point | 20.2 °C |
| Melting Point | -123.5 °C |
Preparation of Acetaldehyde – CH3CHO
1. Oxidation of Ethyl alcohol
Ethyl alcohol (CH3CH2OH) undergoes oxidation in the presence of acidified K2Cr2O7 or aqueous/alkaline KMnO4 produces acetaldehyde.
\(\begin{array}{l} CH_{3}CH_{2}OH + [O] \overset{oxidation}{\rightarrow} CH_{3}CHO + H_{2}O\end{array} \)
2. Catalytic Dehydrogenation of ethyl alcohol.
Ethyl alcohol undergoes catalytic dehydrogenation in presence of a copper catalyst at 573 K and produces acetaldehyde.
\(\begin{array}{l}CH_{3}CH_{2}OH \overset{Cu/573K}{\rightarrow} CH_{3}CHO + H_{2}\end{array} \)
3. Rosenmund’s Reduction.
Acetaldehyde can be prepared by the reduction of acetyl chloride with H2 in the presence of palladium deposited over barium sulphate along with a small amount of salt of sulphur or quinoline. This mixture is known as Lindlar’s catalyst. This reaction is called Rosenmund’s Reduction.
\(\begin{array}{l} CH_{3}COCl \xrightarrow[S/quinoline]{Pd/BaSO_{4}}CH_{3}CHO + HCl\end{array} \)
4. By Wacker Process
Ethene can be converted to acetaldehyde by reacting with an aqueous solution containing Palladium chloride and cupric chloride.
\(\begin{array}{l}CH_{2}= CH_{2} + PdCl_{2} + H_{2}O \overset{CuCl_{2}}{\rightarrow}CH_{3}CHO + Pd + 2HCl\end{array} \)
In this reaction, CuCl2 Converted Pd back into PdCl2.
2CuCl2 + Pd → PdCl2 + 2CuCl
Cuprous chloride is reoxidised to CuCl2 by atmospheric oxygen in the presence of HCl.
4CuCl + 4HCl +O2 → 4CuCl2 + 2H2O
5. Preparation from Acetylene:
Acetylene gets hydrated in the presence of a mixture of diluted H2SO4 and mercuric acid to vinyl alcohol which tautomerises to a more stable compound of acetaldehyde.
CH≡CH + H2O → CH2=CHOH → CH3CHO
Chemical Properties of Acetaldehyde – CH3CHO
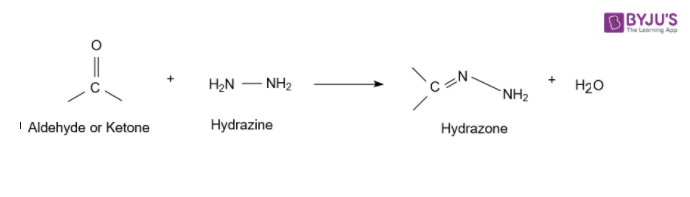
1. Acetaldehyde reacts with Hydrazine
The nitrogen atom of phenylhydrazine acts as a nucleophile which attacks the electrophilic carbon of acetaldehyde and removes water to give acetaldehyde phenylhydrazone.
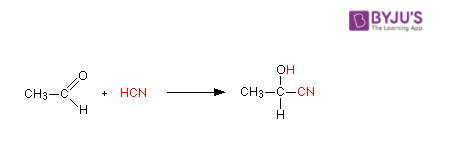
2. Acetaldehyde reacts with HCN
Hydrogen cyanide is added across the carbon-oxygen double bond in aldehydes and ketones to produce compounds known as hydroxynitriles. For example, with ethanal (an aldehyde) you get 2-hydroxypropanenitrile.
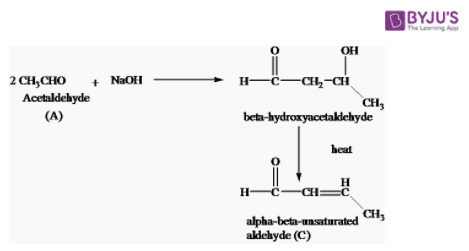
3. Acetaldehyde reacts with NaOH
Acetaldehyde (A) reacts when NaOH undergoes aldol condensation forming beta-hydroxyacetaldehyde on heating giving alpha-beta-unsaturated aldehyde.
Acetaldehyde (C2H4O) Uses
- It was used as a precursor to acetic acid.
- It is used as a precursor to pyridine derivatives, crotonaldehyde, and pentaerythritol.
- Used in the manufacturing of resin.
- It is used to produce polyvinyl acetate.
- It is used in the manufacturing of disinfectants, perfumes, and drugs
- It is used in the production of chemicals such as acetic acid.
Acetaldehyde Toxicity
Acetaldehyde is a significantly more potent toxin than ethanol, and its first metabolite, acetaldehyde, is responsible for at least a portion of ethanol toxicity. Some of the acetaldehyde gets into your bloodstream, causing damage to your membranes and possibly scar tissue. It also causes a hangover, as well as a racing heart, a headache, and an upset stomach. Acetaldehyde intoxication has the greatest impact on the brain. It impairs memory and causes issues with brain activity.
Based on insufficient human cancer research and animal studies that showed nasal cancers in rats and laryngeal tumours in hamsters, acetaldehyde is classified as a potential human carcinogen. One of the main culprits mediating the fibrogenic and mutagenic effects of alcohol in the liver is acetaldehyde, a significant toxic metabolite. Acetaldehyde stimulates the development of adducts, which causes functional impairments in essential proteins, such as enzymes, as well as DNA damage, which promotes mutagenesis.
Frequently Asked Questions – FAQs
What is acetaldehyde?
Acetaldehyde is present in various plants, ripe fruits, vegetables, smoke from tobacco, gasoline and exhaust from the engine. This material is commonly used as a flavouring agent and as an intermediate in alcohol metabolism in the manufacture of acetic acid, perfumes, dyes, and medicines. The chemical formula of Acetaldehyde is CH3CHO
What are the health effects of acetaldehyde?
Acute (short-term) exposure to acetaldehyde results in effects which include eye, skin, and respiratory tract irritation. Symptoms of chronic (long-term) acetaldehyde poisoning mimic those of alcoholism.
How does acetaldehyde affect the liver?
A major toxic metabolite, acetaldehyde is one of the main culprits mediating fibrogenic and mutagenic effects of alcohol in the liver. Mechanistically, acetaldehyde promotes the formation of adducts, leading to functional impairments of key proteins, including enzymes, and DNA damage, which promotes mutagenesis.
What is the difference between formaldehyde and acetaldehyde?
The iodoform test will differentiate the formaldehyde and acetaldehyde. Methyl ketones react to yellow precipitate by giving iodine and potassium hydroxide. Acetaldehyde binds to the carboxylic acid with iodine and KOH to give sodium salt. Formaldehyde does not screen for iodoformity.
What are the uses of acetaldehyde?
In other applications, acetaldehyde serves as an additive such as fruit and fish preservatives, flavouring agents, and gelatine hardening. Acetaldehyde is also used as a preservative for fruit and fish in the manufacture of vinegar, yeast and.
Will acetaldehyde pass the Tollen’s Test?
What is the key difference between acetaldehyde and acetone?
Can acetaldehyde act as an excellent reducing agent?
Can acetaldehyde cause an iodoform test to be positive?
CnH2n+1CHO is the general formula for aldehydes. What is the “n” value for acetaldehyde?
Learn more about the Structure, physical and chemical properties of Acetaldehyde from the experts at BYJU’S.
Other related links:
| Oxidizing Agent | Potassium Permanganate |

Comments