What is Acetone?
Acetone, commonly known as Propanone, is a colourless liquid used in the production of plastics and other industrial products.
Acetone formula (or Propanone) is given here both in organic form and in structural form. To recall, acetone is the smallest and the simplest Ketone which is flammable, colourless and volatile liquid. It is an organic compound and is also known as Propanone. Learn more about acetone, its structure, and properties in the linked article.
Table of Contents
- Acetone Formula
- Structural Formula of Acetone
- Organic Formula of Acetone
- Properties of Acetone
- Uses of Acetone
- Frequently Asked Questions – FAQs
Acetone Formula
Both the structural formula and the organic formula of acetone with its structure is given below. The structure will help to understand this compound better and help to understand the organic formula better.
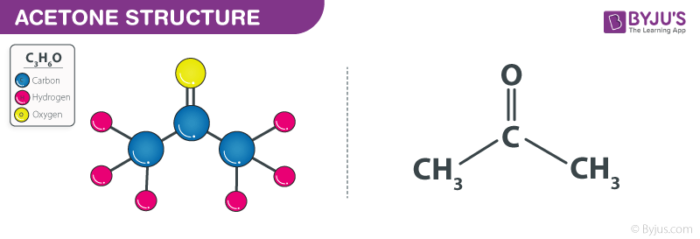
Structural Formula of Acetone (Propanone)
Organic Formula (Chemical Formula) of Acetone
Acetone consists of three carbon atoms, six hydrogen atoms, and one oxygen atom. It is considered as a ketone since there is a carbonyl group present in it. Thus, the chemical formula for acetone or propanone is written as-
| Acetone Chemical Formula = C3H6O |
Acetone is mainly used in medicine and in cosmetics. Acetone is present in blood and in urine. Acetone is also an active ingredient in nail polish removers. Visit uses of acetone to learn more about its uses in different sectors.
Properties of Acetone
| Acetone | C3H6O |
| Appearance | Colourless liquid |
| Odor | Pungent, irritating, floral |
| Boiling Point | 56oC |
| Melting Point | -95oC |
| Molecular mass | 58.08 g/mol |
| Density | 0.7845 g/cm3 (25 °C) |
Uses of Acetone
- Acetone is used in different fields. Some of them are listed below.
- Medicine or pharmaceutical industry
- Used in cosmetics
- Used in laboratory
- Used in electronics
- Used for domestic purposes
Read more: Acetone Uses
Frequently Asked Questions – FAQs
What acetone is used for?
Acetone is a chemical used to make products like nail polish remover and paint remover. Your body also makes this chemical when it breaks down fat.
Is acetone harmful to humans?
Inhaling a moderate amount of acetone in a short period of time can irritate the nose, throat, lungs and eyes. Also affected by headache, dizziness, confusion, tachycardia, nausea, vomiting, blood, the possibility of fainting and coma can cause a shortened menstrual cycle in women.
What is the chemical name for acetone?
The simplest and most important of the acetone (CH3COCH3), also known as propanone or dimethyl ketone, industrial, chemically important organic solvents, and aliphatic (fat-derived) ketones.
Is acetone heavier than water?
Acetone exists as a liquid at room temperature, but at room temperature, it is less dense than about 1 g/mL of water. The density of acetone is 0.788g/mL at room temperature. This means that per millilitre of the liquid has a mass of 0.788 grams.
Is acetone a better solvent than water?
Acetone is a good solvent because of its ability to dissolve both polar and nonpolar substances, but other solvents can dissolve either. Second, acetone is a good solvent because it is a miscible substance. That is, it has the ability to mix with water in all proportions.
How many protons are chemically equivalent in acetone?
How many 1H NMR signals would acetone produce?
Can acetone pass the Schiff’s test?
Schiff’s reagent is the end product of a reaction between a dye and sodium bisulfite. Acetone will not pass the Schiff test because it contains ketone as a functional group.

Comments