What are Aldehydes, Ketones, and Carboxylic Acids?
Aldehydes, Ketones, and Carboxylic Acids are carbonyl compounds which contain a carbon-oxygen double bond. These organic compounds are very important in the field of organic chemistry and also have many industrial applications.
Table of Contents
- Recommended Videos
- What are Aldehydes?
- Preparation of Aldehydes
- Properties of Aldehydes
- Nomenclature of Aldehydes
- Uses of Aldehydes
- What are Ketones?
- Preparation of Ketones
- Properties of Ketones
- Nomenclature of Ketones
- Uses of Ketones
- What is Carboxylic Acids?
- Preparation of Carboxylic Acids
- Properties of Carboxylic Acids
- Nomenclature of Carboxylic Acids
- Frequently Asked Questions – FAQs
The presence of the common carbonyl group in the two classes of compounds makes them display similar chemical properties. However, aldehydes are more reactive than ketones because of the presence of free hydrogen atom.
Organic compounds containing a carbon-oxygen double bond called the carboxyl group, which is one of the most important functional groups in organic chemistry. Carbonyl group is one of the important groups present in compounds in a living system.
Recommended Videos
What affects Carboxylic Acid Acidity?
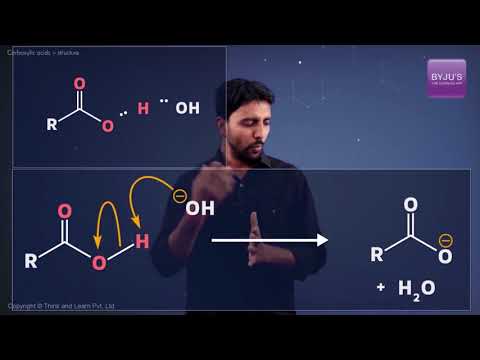
Functional Group in Aldehydes and Ketones

Carbonyl Compounds – Important and JEE PYQs

Carbonyl Compounds – Important Topics for JEE

What are Aldehydes?
Aldehydes are organic compounds which have the functional group -CHO.
These carbonyl compounds consist of a central carbonyl carbon doubly bonded to an oxygen and single bonded to the R group (any alkyl group) and a hydrogen atom.
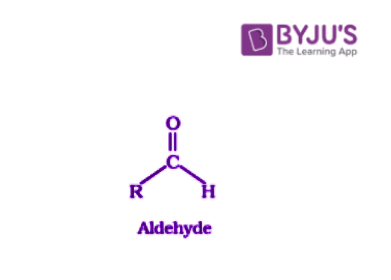
Where R stands for alkyl or aryl group.
Preparation of Aldehydes
Acid chlorides are reduced to aldehydes with hydrogen in the presence of palladium catalyst spread on barium sulfate.
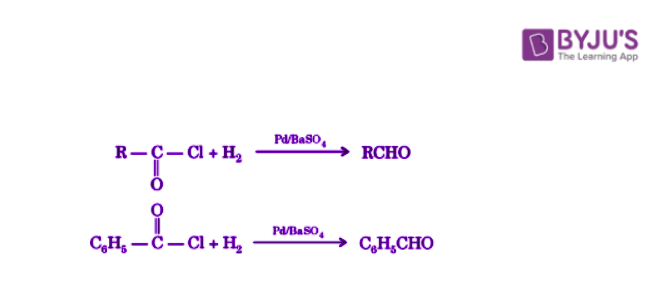
This reaction is known as Rosenmund reduction and is mostly used for the preparation of aromatic aldehydes. This reaction cannot be used for the preparation of ketones and formaldehyde.
Properties of Aldehydes
- The structure of aldehydes shows a sp2 hybridized central carbon which is doubly connected to oxygen and has a single bond with hydrogen.
- Small aldehydes are quite soluble in water.
- Formaldehyde and acetaldehyde are great examples of this. These two aldehydes are quite important industrially.
- Aldehydes generally exhibit a tendency to undergo oligomerization or polymerization.
- The carbonyl centre of the aldehyde has an electron-withdrawing nature. Therefore, the aldehyde group can be considered somewhat polar.
Nomenclature of Aldehydes
- Acyclic and Aliphatic Aldehydes are named after their longest carbon chain along with the “-al” suffix. For example, CH3CH3CH2CHO is called butanal since it has four carbons in the chain.
- When the aldehyde functional group is added to a ring, the “-carbaldehyde” suffix must be used. For example, C6H11CHO is called cyclohexanecarbaldehyde.
- In the case of natural compounds or carboxylic acids, “-oxo” is used as a prefix to highlight the carbon which is a part of the aldehyde functional group. For example, (CHO)-CH2COOH is called 3-oxopropanoic acid.
Some common and IUPAC names for some aldehydes are tabulated below.
| Formula | Common name | IUPAC name |
| HCHO | Formaldehyde | Methanal |
| CH3CHO | Acetaldehyde | Ethanal |
| CH3-CH(CH3)-CHO | Isobutyraldehyde | 2-Methylpropanal |
| CH3-CH=CH-CHO | Crotonaldehyde | 2-Butenal |
Uses of Aldehydes
- Formaldehyde is used as a disinfectant and as a preservative for biological specimens.
- Aldehyde is used for silvering mirrors.
- Formaldehyde is used for the production of a variety of plastic and resins.
- Benzaldehyde is used in perfumery and in the dye industry.
What are Ketones?
Ketones are organic compounds which have the functional group C=O and the structure R-(C=O)-R’.
These carbonyl compounds have carbon-containing substituents on both sides of the carbon-oxygen double bond. The carbonyl carbon of the ketone group is sp2 hybridized. The structure of ketones is a trigonal planar centred around the carbonyl carbon. The bond angles of this structure approximate at 1200. Since the carbon-oxygen bond makes the carbonyl group polar (oxygen is more electron-withdrawing than carbon), ketones tend to be nucleophilic at the oxygen atom and electrophilic at the carbon atom.
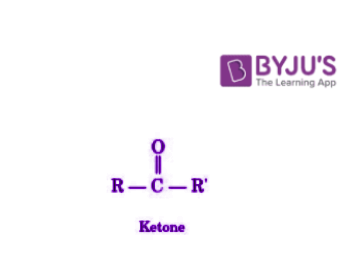
Ketones are mass-produced industrially for their use as solvents, pharmaceuticals, and as precursors for polymers. Important ketones include methyl ethyl ketone (also called butanone), cyclohexanone, and acetone.
Preparation of Ketones
Acid chlorides on reaction with dialkyl cadmium produce ketones. Dialkyl cadmium themselves are prepared from Grignard reagents.
2R-Mg-X + CdCl2 → R2Cd + 2 Mg(X)Cl
2RCOCl + R2Cd → 2R-CO-R + CdCl2
The method is useful in a way that the mixed ketones can be prepared very conveniently.
Properties of Ketones
- Ketones are polar in nature due to the presence of a polar carbonyl group. Therefore they have higher boiling points than non-polar compounds.
- It cannot form any intermolecular hydrogen bond-like alcohols because there is no hydrogen attached to an oxygen atom.
- Ketones have large dipole moments compared to alcohols or ethers due to the shifting of pi electrons.
- Ketones react with hydrogen cyanide to form cyanohydrins. The reaction is normally carried out in the presence of a base, which acts as a catalyst in the absence of a base the reaction proceeds slowly.
- Most of the ketones form bisulphite addition products when it is added to sodium bisulphite.
Nomenclature of Ketones
- Ketones are named after their parent alkanes with the suffix “-anone”. The carbonyl group’s position in the ketone is denoted by a number while naming the ketone. For example, CH3(CO)CH3 is called 2 propanone. However, this compound is generally referred to as acetone.
- Commonly, ketones are named by writing the name of each individual alkyl group attached to the carbonyl carbon and then “ketone” as the third word of the name. For example, butanone can be written as methyl ethyl ketone.
| Formula | Common name | IUPAC name |
| CH3-C(O)-CH3 | Acetone | Propanone |
| CH3-C(O)-CH2-CH3 | Ethylmethylketone | Butanone |
| CH3-CH2-C(O)-CH2-CH3 | Diethyl ketone | Pentan-3-one |
| CH3-CH(CH3)-C(O)-CH3 | Isopropyl methyl ketone | 3-Methylbutan-2-one |
Uses of Ketones
- Propanone is used to make polymers for example perspex.
- ketones are used as solvents and as a starting material for the synthesis of many organic compounds.
- Acetone and ethyl methyl ketone is mainly used as industrial solvents.
What is Carboxylic Acid?
Carboxylic acids are organic compounds that contain a (C=O)OH group attached to an R group (where R refers to the remaining part of the molecule).
The COOH group is commonly referred to as a carboxyl group. Carboxylic acids can generally be expressed via the formula R-COOH. Carboxylic acids have a polar nature. They can also participate in hydrogen bonding owing to their hydrogen bond accepting nature of the C=O group and the hydrogen bond donating nature of the O-H bond. They generally have higher boiling points when compared to water and tend to form stable dimers.
Carboxylic acids play an important role in the production of pharmaceuticals, food additives, solvents, and polymers. Acetic acid, adipic acid, and citric acid are a few carboxylic acids which are extremely useful industrially.
Preparation of Carboxylic Acids
Primary alcohols are readily oxidized to carboxylic acids with common oxidizing agents such as potassium permanganate in neutral acidic or alkaline media or by potassium dichromate and chromium trioxide in acidic media.
RCH2OH → RCOOH
CH3(CH2)8CH2OH → CH3(CH2)8COOH
Properties of Carboxylic Acids
- Carboxylic acids are polar compounds and can extensively enter into hydrogen bonding.
- Aromatic carboxylic acids are practically insoluble in cold water. All carboxylic acids are soluble in organic solvents like ether, alcohol, benzene, etc.
- Among organic acids, carboxylic acids are the most acidic, but they are less acidic than the mineral acids, namely nitric acid and sulphuric acid.
Nomenclature of Carboxylic Acids
- Carboxylic acids are named by adding an “-oic acid” suffix to their parent chain. For example, C3H7COOH is called butanoic acid.
- Even if there are other substituents present, the carboxylic acid can be considered at the first position of the parent chain, as seen in the name for 3-Chloropropanoic acid.
- The COOH group can also be called “carboxy” and used as a substituent in the name of the parent structure. For example, 2-Furoic acid is commonly referred to as 2-carboxy furan.
| Formula | Common name | IUPAC name |
| HCOOH | Formic acid | Ethanoic acid |
| CH3COOH | Acetic acid | Ethanoic acid |
| CH3CH2COOH | Propionic acid | Propanoic acid |
| C6H5COOH | Benzoic acid | Benzenecarboxylic acid |
Frequently Asked Questions – FAQs
Do carboxylic acids react with aldehydes?
The carbonyl groups in aldehydes and ketones can be oxidized to form the next “oxidation level” compound-carboxylic acid. Adding water to an aldehyde or ketone produces a product called a hydrate or gemdiol (two OH groups on one carbon). The reaction is catalyzed by acids and bases.
Are ketones and aldehydes carboxylic acid derivatives?
The difference between carboxylic acid derivatives and aldehydes and ketones is that there is a group containing a negatively charged heteroatom (usually oxygen, nitrogen or sulfur), which is directly connected to the carbonyl carbon atom. You can think of carboxylic acid derivatives as bilateral.
Which is more acidic aldehyde or ketone?
Compared with the alkyl groups of ketones, aldehydes are more acidic (lower pKa) than ketones due to the lower electron donating effect of protons.
What does Schiff’s test for?
The Schiff test is a chemical test used to check the presence of aldehydes in a specific analyte by reacting the analyte with a small amount of Schiff reagent.
How do you purify aldehydes?
The solid aldehyde can be dissolved in ether and purified as described above. Alternatively, they can be steam distilled, then sublimated and crystallized from toluene or petroleum ether.

Comments