What is Acidity Of Alkynes?
Alkynes are one of the simplest hydrocarbons known to us. They have a general formula of CnH2n-2.
Alkynes belong to the family of unsaturated hydrocarbons that is; they contain both sigma and pi bond linkages between carbon and hydrogen.
Table of Contents
- Acidity Of Alkynes Explanation
- Relative Acidity of Alkynes
- Recommended Videos
- Frequently Asked Questions – FAQs
Acidity Of Alkynes Explanation
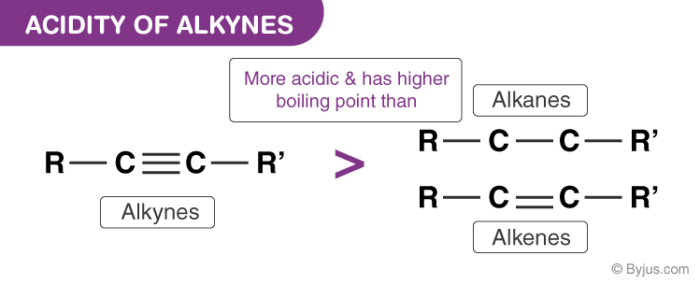
An alkyne molecule contains at least one triple bond between two carbon atoms. For example ethyne (CH≡CH). Ethyne reacts with strong bases such as sodium metal and sodamide (NaNH2)to form sodium acetylide along with the liberation of dihydrogen gas. This reaction of alkynes with bases to liberate dihydrogen gas indicates the acidity of alkynes.
HC ≡ CH + Na → HC ≡ C– Na+ + 1/2H2
Relative Acidity of Alkynes
Acidity of alkynes is due to their ability to lose hydrogen atom to form alkynideions. Thus, alkynes act as Brønsted-Lowry acids. The triple bonded carbon atom in alkynes is “sp” hybridized. Due to the high percentage of “s” character (50%) in alkynes, the “sp” hybridized orbitals of carbon atom in alkynes exhibit high electronegativity. These attract the C-H bond of alkynes to a great extent. Thus, alkyne molecules can easily lose hydrogen atom forming alkynide ions. Hence, we can say that the hydrogen atom attached to the triply bonded carbon atom is acidic in nature.
Click here to know about Physical Property of Alkynes
The acidity of alkynes is greater than the acidity of alkanes and alkenes as the carbon atom in alkanes and alkenes are “sp3” and “sp2” hybridized respectively. Hence, these molecules contain a smaller percentage of “s” character in comparison to alkynes. Thus, the electronegativity of the carbon atom in these cases is lesser than alkynes. Hence, alkanes and alkenes don’t show the reactions with bases to liberate hydrogen gas. It can further be noted that only hydrogen atom attached to a triply bonded carbon atom are acidic not the other hydrogen atoms in the alkyne chain. The general trend in acidity is seen as:
HC≡CH > H2C=CH2> CH3–CH3
HC≡CH>CH3–C≡CH>>CH3–C≡C–CH3
For a detailed discussion on the acidity of alkynes and other topics related to acids and bases, such as di and polybasic acids and bases, download BYJU’S – the learning app.
Recommended Videos
Test for Terminal Alkynes

Frequently Asked Questions – FAQs
Are alkynes strong acids?
1-Alkynes are very weak acids, consequently their conjugate bases, RC≡C⊖, are pretty sturdy bases. These anions are also reactive carbon nucleophiles, and it’s miles this assets that makes them beneficial for natural synthesis.
Are all alkynes acidic?
Alkynes are slightly acidic. Exposure to strong bases such as sodium amide can cause acid-base reactions. The acidity of the terminal alkynes is due to the high s characteristic of the sp hybrid orbital, which combines with the s orbital of the hydrogen atom to form a simple covalent bond.
What is the cause of acidity of alkynes?
Alkynes are acidic because they can release hydrogen atoms to form alkyne ions. Therefore, the alkyne is used in the form of Brönsted-Lowry acid. As mentioned above, alkynes contain a triple-bonded carbon atom called sp. Hybrid.
Are alkynes more acidic than alcohols?
Alcohol is more acidic than alkynes. For alcohols, H + is removed to form O. When H + is removed, O is more stable than C formed in alkynes. Because the electronegativity of oxygen is greater than that of carbon.
Which hydrocarbon is most acidic?
Cyclopentadiene is actually one of the most acidic hydrocarbons. The hydrogen atoms above have a pKa of 15.

Comments