To determine the presence of nitrate ion in any solution, a chemical test called a nitrate test is conducted. Almost all nitrates dissolve in water and therefore determining the presence of nitrate through wet chemistry is not that easy when compared to conducting tests for other anions. Nitrate anion is an oxidizer, and many tests for the nitrate anion depend on this property. Other oxidants present in the analyte may interfere to give incorrect results.
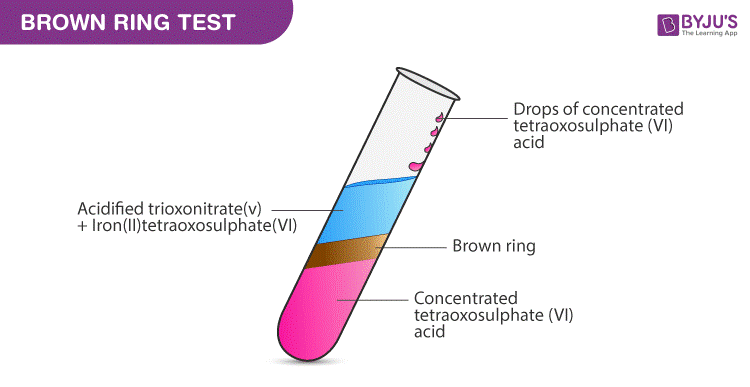
Brown Ring Test Procedure:
A common nitrate test is also known as the brown ring test. The experiment can be performed as follows:
Step 1: Take the solution of nitrate.
Step 2: Add iron(II) sulfate
Step 3: Slowly add concentrated sulphuric acid (H2SO4) such that the acid added forms a layer below the aqueous solution.
Result – A ring brown in color will be formed at the junction of the 2 layers. This indicates the presence of nitrate ion.
Brown Ring Test Reaction:
The overall reaction is given below:
Reduction of the nitrate ion, oxidation of iron(II) and reduction of nitric oxide.
2HNO3+ 3H2SO4 + 6FeSO4 —>> 3Fe2(SO4)3 + 2NO + 4H2O
(Remaining) [Fe(H2O)6]SO4 + NO = [Fe(H2O)5(NO)]SO4+ H2O
This test is quite sensitive till 2.5 micrograms and a concentration of one in twenty-five thousand parts.
To learn more about the brown ring test (nitrate test) from the expert faculties at BYJU’S, register now! Also, for the latest updates on chemistry study materials such as notes, question papers, etc. keep visiting us.
Other important links:

Good