What is Brownian Motion?
“Brownian motion refers to the random movement displayed by small particles that are suspended in fluids. It is commonly referred to as Brownian movement”. This motion is a result of the collisions of the particles with other fast-moving particles in the fluid.
Brownian motion is named after the Scottish Botanist Robert Brown, who first observed that pollen grains move in random directions when placed in water. An illustration describing the random movement of fluid particles (caused by the collisions between these particles) is provided below.
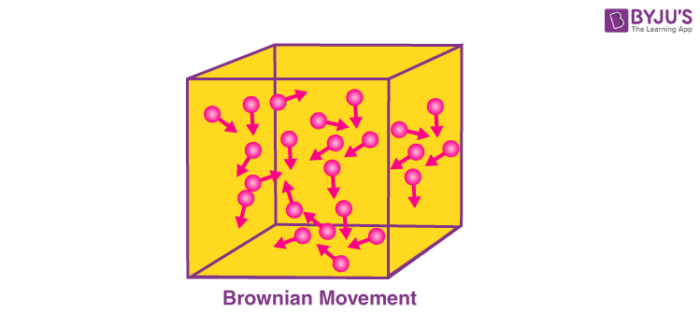
A particle changes its path when another particle collides with it. Further collisions cause the particle to follow a random, zig-zag motion. It involves a transfer or exchange of momentum/energy between the particles.
Causes and Effects of Brownian Motion
Brownian motion describes randomness and chaos. It is one of the simplest models of randomness. The various causes and effects of this motion are listed in this subsection.
1. What Causes Brownian Motion?
- The size of the particles is inversely proportional to the speed of the motion, i.e. Small particles exhibit faster movements.
- This is because the transfer of momentum is inversely proportional to the mass of the particles. Lighter particles obtain greater speeds from collisions.
- The speed of the Brownian motion is inversely proportional to the viscosity of the fluid. The lower the viscosity of the fluid, the faster the Brownian movement.
- Viscosity is a quantity that expresses the magnitude of the internal friction in a liquid. It is the measure of the fluid’s resistance to flow.
2. Effects of Brownian Motion
- Brownian movement causes the particles in a fluid to be in constant motion.
- This prevents particles from settling down, leading to the stability of colloidal solutions.
- A true solution can be distinguished from a colloid with the help of this motion.
Albert Einstein’s paper on Brownian motion was vital evidence on the existence of atoms and molecules. The kinetic theory of gases which explains the pressure, temperature, and volume of gases is based on the Brownian motion model of particles. To learn more about this topic and other related topics, such as entropy, register with BYJU’S and download the mobile application on your smartphone.

Nice. Crystal clearance
Nice 👍👍