What is Carbolic Acid?
Carbolic acid (commonly known as phenol) is an aromatic chemical molecule with the molecular formula C6H5OH and the molecular formula C6H5OH. It is a flammable white crystalline substance. Carbolic acid is the simplest member of the phenol family of organic compounds.
Phenols are sometimes known as carbolic acids because of their extreme acidity. Because of resonance, the phenol molecule has a partial positive charge on the oxygen atom, and the anion created by the loss of a hydrogen ion is similarly resonance stabilised. Phenol is hydroxybenzene by definition. The substance is known by the name phenol. The IUPAC name for it would be benzenol, which was derived similarly to the IUPAC names for aliphatic alcohols.
Table of Contents
- Carbolic Acid Formula
- Carbolic Acid Structure
- Carbolic Acid Properties
- Carbolic Acid Uses
- Frequently Asked Questions – FAQs
Carbolic Acid Formula
The formula for carbolic acid is C6H5OH.
Carbolic acid, also known as carbolic acid and phenolic acid, is a colourless, white crystalline solid at ambient temperature that is found naturally.
Carboxylic acids with a low molecular weight are usually liquids or solids with a low melting point. Most low molecular weight phenols are water-soluble due to hydrogen bonding. Because of stronger intermolecular hydrogen bonding, phenols have higher boiling temperatures than alcohols of the same molecular weight.
Carbolic Acid Structure
Phenol, also known as carbolic acid is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile.
The structure of Carbolic acid (phenol) is given below.
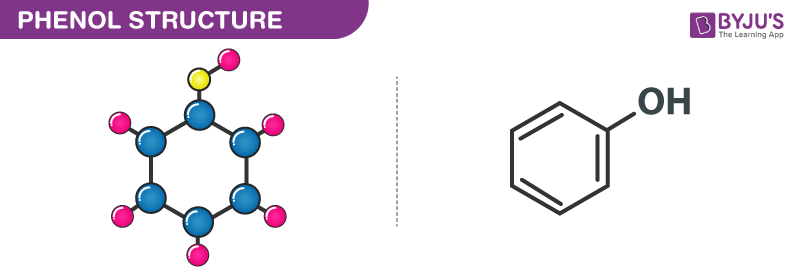
Carbolic Acid Properties
Physical Properties of Carbolic Acid
- Carbolic acid is a natural product.
- Carbolic acid is water-soluble.
- Carbolic acid is a mildly acidic substance.
- The carbolic acid is transformed into the phenolate ion in an aqueous medium.
- Carbolic acid has resonance properties.
- Hydrogen bonds are formed by carbolic acid.
- Tautomerism is a property of carbolic acid.
Chemical Properties of Carbolic Acid
1. Addition of base to the phenol
Phenol reacts with: A base (like NaOH) to form the phenoxide anion. This is a deprotonation reaction, due to the removal of the proton (hydrogen).
C6H5OH + NaOH → C6H5ONa + H2O
2. Addition of Acyl Group
A typical acyl chloride is ethanoyl chloride, CH3COCl. Phenol reacts with ethanoyl chloride at room temperature, although the reaction isn’t as fast as the one between ethanoyl chloride and alcohol. Phenyl ethanoate is formed together with hydrogen chloride gas.
C6H5OH + C6H5COCl → C6H5OCOC6H5 + HCl
3. Reduction under zinc dust
Distillation of phenol with zinc-dust gives benzene and ZnO as a side-product.
C6H5OH + Zn → C6H6 + ZnO
4. Oxidation of phenol
Oxidation of phenols gives benzoquinone. Phenols are more readily oxidised than alcohols. Oxidation can be done by using strong oxidising agents like chromic acid, silver oxide, etc. When phenol is oxidised using chromic acid in presence of sulphuric acid and water it forms benzoquinone.
Carbolic Acid Uses
The use of carbolic acid on metal surfaces at high temperatures increases the risk of corrosion on steel. The corrosion rate of low-carbon steel has been observed to be reduced when metal surfaces are exposed to carbolic acid and moisture in the 0.2 percent to 0.6 percent range.
However, when the moisture level rose, so did the rate of deterioration. Corrosion of low-carbon steel is substantially accelerated at temperatures exceeding 200°C. Furthermore, carbolic acid containing substantial sulphur is more corrosive to certain metals than pure carbolic acid.
Plastic precursors are the most prevalent usage of phenol, accounting for two-thirds of its total use. Bisphenol-A, a major precursor to polycarbonates and epoxide resins, is produced by condensation of bisphenol-A with acetone. phenolic resins, such as bakelite, are generated when phenol, alkylphenols, or diphenols react with formaldehyde to form phenolic resins. When phenol is partially hydrogenated, cyclohexanone is generated, which is a precursor to nylon. Nonylphenol is produced via alkylation of phenol, which is subsequently ethoxylated to produce nonionic detergents.
Frequently Asked Questions on Carbolic Acid
What is carbolic acid used for?
Phenol (carbolic acid) is one of the oldest antiseptic agents. Apart from being used in many commercially available products, in rural India, it is often used in the household to prevent snake infestation.
Are phenol and carbolic acid the same?
Yes, phenol which is a colourless, white crystalline solid at room temperature is also known as carbolic acid
What is hydroxy benzene used for?
A caustic, poisonous, white crystalline compound, C6H5OH, derived from benzene and used in resins, plastics, and pharmaceuticals and in dilute form as a disinfectant and antiseptic.
What is resorcinol made of?
Resorcinol, also called m-dihydroxybenzene, is a phenolic compound used in the manufacture of resins, plastics, dyes, medicine, and numerous other organic chemical compounds. It is produced in large quantities by sulfonating benzene with fuming sulfuric acid and fusing the resulting benzenedisulfonic acid with caustic soda.
What is naphthol used for?
The compound 1-naphthol, or α-naphthol, made by heating 1-naphthalenesulfonic acid with caustic alkali or by heating 1-naphthylamine with water under pressure, is used directly in making several dyes, and large amounts of it are converted to compounds ultimately incorporated into other dyes.
Comments