Carbon dioxide Definition
One carbon atom and two oxygen atoms make up carbon dioxide, a chemical compound. The formula CO2 is often used to refer to it.
Carbon dioxide is a chemical element that can be found in the atmosphere. At room temperature, it is a gas. It has one carbon atom and two oxygen atoms. When people and animals exhale, carbon dioxide is released. It is a greenhouse gas that is found in low concentrations in the Earth’s atmosphere. Dry ice is what it is when it is firm.
Human activities contribute carbon dioxide to the atmosphere. Carbon dioxide is emitted when hydrocarbon fuels (such as wood, coal, natural gas, gasoline, and oil) are burned. Carbon from fossil fuels reacts with oxygen in the air to form carbon dioxide and water vapour during combustion or burning.
Carbon dioxide Structure
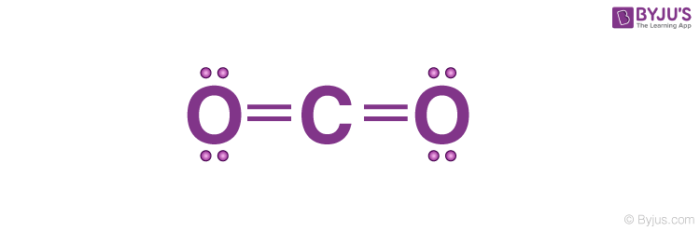
Properties of Carbon dioxide
Carbon dioxide is a gas that is both colourless and odourless. Water, ethanol, and acetone are all soluble in it. Some general properties of carbon dioxide are given below.
| Molecular formula | CO2 |
| Molar mass | 44.0095(14) g/mol |
| Density | 1,600 g/L – solid
771 g/L – liquid 1.98 g/L – gas |
| Melting point | −56.6 °C |
| Boiling point | −78.5 °C |
| Specific gravity | 1.53 at 21oC |
| Synonyms | Carbonic anhydride
Dry ice Carbonic acid gas |
| Henry constant for solubility | 298.15 mol/ kg * bar |
| Water solubility | 0.9 vol/vol at 20oC |
Chemical Properties of Carbon dioxide
According to the following reaction, carbon dioxide dissolves slightly in water to form a weak acid called carbonic acid, H2CO3:
CO2 + H2O → H2CO3
The hydronium cation, H3O+, and the bicarbonate ion, HCO3–, are formed after carbonic acid reacts slightly and reversibly in water, as follows:
H2CO3 + H2O → HCO3– + H3O+
Plants take in carbon dioxide (CO2) and water (H2O) from the air and soil during photosynthesis. Water is oxidized in the plant cell, which means it loses electrons, while carbon dioxide is reduced, which means it gains electrons. Water is converted to oxygen, and carbon dioxide is converted to glucose. The plant then releases the oxygen into the atmosphere while storing energy in the glucose molecules.
The mechanism by which green plants and other organisms convert light energy into chemical energy is known as photosynthesis. Light energy is absorbed and used by green plants during photosynthesis to turn water, carbon dioxide, and minerals into oxygen and energy-rich organic compounds.
Main Function of Carbon dioxide
Proteins, lipids, nucleic acids, and carbohydrates all contain carbon as their primary part. Carbon’s molecular structure allows it to bond with a variety of elements in a variety of ways. The carbon cycle depicts how carbon passes through the environment’s living and non-living components.
Carbon dioxide (CO2) is the primary greenhouse gas emitted through human activities. Proteins, lipids, nucleic acids, and carbohydrates are all made up of carbon as the primary part. Carbon’s molecular structure allows it to form bonds with a variety of elements in a variety of ways. The carbon cycle depicts the movement of carbon in the world, both living and non-living.
Photosynthesis and respiration, two essential plant and animal processes, both require carbon dioxide. Carbon dioxide and water are converted into food compounds such as glucose and oxygen by green plants. Photosynthesis is the name for this process.
The reaction of photosynthesis is as follows:
6CO2 + 6H2O → C6H12O6 + 6O2
Carbon dioxide is the body’s primary hormone; it is the only one that is released by every tissue and likely works on every organ. CO2 serves a variety of roles in the body, including oxygen delivery to cells, blood pH regulation, and much more.
Recommended Videos

Environmental Problems – Carbon dioxide
- A rise in carbon dioxide levels results in an overabundance of greenhouse gases, which trap more heat. This trapped heat causes ice caps to melt and ocean levels to rise, causing flooding.
- CO2 emissions contaminate our clean air, forming an impenetrable layer across the globe. This sheet, in a sense, traps the heat inside the planet, causing global warming. The Greenhouse effect is another name for this operation.
- Carbon dioxide is released into the environment by burning fossil fuels, releasing chemicals into the atmosphere, reducing forest cover, and the rapid growth of cultivation, production, and industrial activities, both of which change the climate system’s equilibrium.
- The temperature of the Earth is determined by a balance between incoming solar energy and energy reflected back into space. The heat that would otherwise be lost to space is absorbed by carbon dioxide. Any of this energy is re-emitted to Earth, causing the atmosphere to become even hotter.
Frequently Asked Questions – FAQs
What is carbon dioxide in the body?
CO2 (carbon dioxide) is a colourless and odourless gas. It’s a waste product that our body produces. Carbon dioxide is carried to our lungs by our blood. Without thinking about it, we exhale carbon dioxide and inhale oxygen all day, every day. The level of carbon dioxide in your blood is measured by a CO2 blood test.
What are the benefits of carbon dioxide?
Increased carbon dioxide concentrations increase photosynthesis, which promotes plant growth, according to studies. Although increased carbon dioxide levels in the air are beneficial to plants, they are also the primary cause of climate change.
What are 3 uses for carbon?
Carbon is used as a fuel (in the form of coal, which is mostly carbon). Pencil tips, high-temperature crucibles, dry cells, electrodes, and lubricants are all made of graphite. Diamonds are used in jewellery as well as in industry for cutting, drilling, grinding, and polishing due to their extreme hardness.
Why is carbon dioxide important to the human body?
Internal respiration in the human body requires carbon dioxide. Internal respiration is a mechanism that transports oxygen to body tissues while also transporting carbon dioxide away from them. Carbon dioxide is a protector of the blood’s pH, which is essential for life.
What are the physical properties of carbon dioxide?
Carbon dioxide has no colour. The gas is odourless at low concentrations but has a strong, acidic odour at sufficiently high concentrations. Carbon dioxide has a density of about 1.98 kg/m3 at normal temperature and pressure, which is around 1.53 times that of air.
Comments