Amines: Amines are one of the most important class of organic compounds which are derived by replacing one or more hydrogen atoms by an alkyl or aryl group in a molecule of ammonia. They are present in vitamins, proteins, hormones, etc. They are extensively used in the manufacturing of many drugs and detergents.
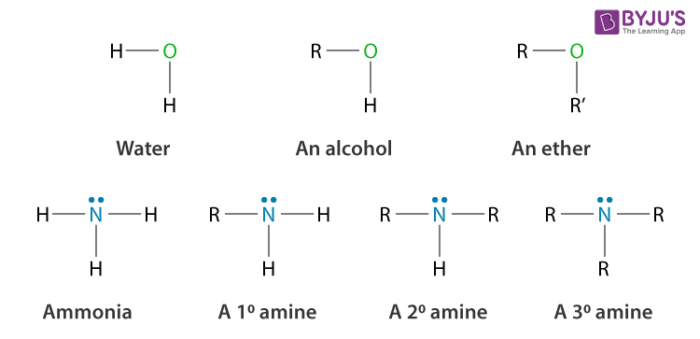
Classification of amines: Depending upon the number of hydrogen atoms that are replaced by an alkyl or aryl group in ammonia, amines are classified as primary (1o), secondary (2o) and tertiary (3o). If only one hydrogen atom is replaced then amines of the form R-NH2 or primary amines (1o) are obtained. If two of the three hydrogen atoms are replaced by alkyl/aryl groups then secondary amines are formed. If all the three hydrogen atoms are replaced by alkyl/aryl group then tertiary amines are obtained.
Nomenclature: In organic chemistry, the names of the compounds that are globally accepted are given according to the guidelines given by IUPAC for the nomenclature of organic compounds. Naming of aliphatic amines is done by prefixing the alkyl group to amines and thus the names of aliphatic amines are of the form of alkylamine. For examples CH3NH2 is named as methylamine (alkyl part + amine =methylamine). Prefixes such as di and tri are appended before the names of the alkyl group when two or more identical groups are present. If more than one amino group is present in the amine then the parent chain and the position of amino groups is identified by numbering the carbon atoms in the parent chain. The numbering is done in such a way that the carbon atom bearing the –NH2 groups get the lowest numbers. Prefixes along with the numbers are then used to denote the number of amino groups and their position in the molecule. For example: H2N-CH2-CH2-NH2 is named as ethane 1, 2-diamine.
If –NH2 group is attached to a benzene ring then it is called as arylamines.
One of the simplest examples of arylamine is C6H5NH2. It is commonly known as aniline which is also an accepted IUPAC name. When we name arylamines according to the guidelines given by IUPAC then ‘e’ of the arene is replaced by amine for example C6H5-NH2 is named as benzenamine.
For more further details download Byju’s- The Learning App.


Comments