What is Column Chromatography?
In chemistry, Column chromatography is a technique which is used to separate a single chemical compound from a mixture dissolved in a fluid.
Column chromatography separates substances based on differential adsorption of compounds to the adsorbent as the compounds move through the column at different rates which allows them to get separated in fractions. This technique can be used on a small scale as well as large scale to purify materials that can be used in future experiments. This method is a type of adsorption chromatography technique.
Table of Content
Column Chromatography Principle
When the mobile phase along with the mixture that needs to be separated is introduced from the top of the column, the movement of the individual components of the mixture is at different rates. The components with lower adsorption and affinity to the stationary phase travel faster when compared to the greater adsorption and affinity with the stationary phase. The components that move fast are removed first whereas the components that move slowly are eluted out last.
The adsorption of solute molecules to the column occurs in a reversible manner. The rate of the movement of the components is expressed as:
Rf = the distance travelled by solute/ the distance travelled by the solvent
Column Chromatography Diagram
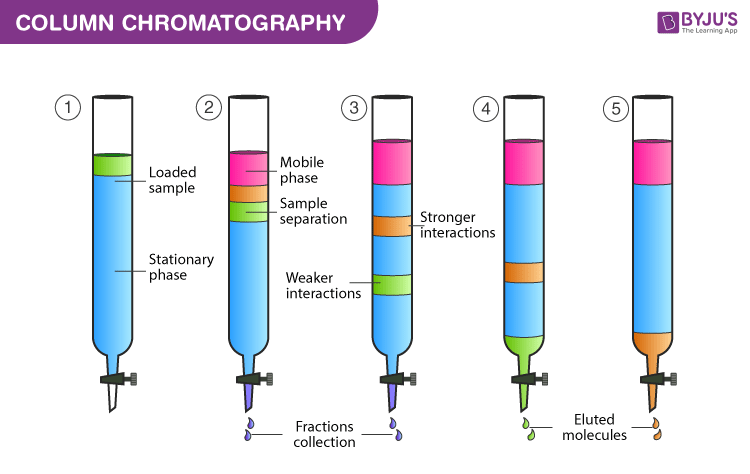
Column Chromatography Diagram
Elution
Elution is a chemical process that involves removing a material’s ions by ion exchange with another material. The chromatographic technique of extracting an adsorbed substance from a solid adsorbing media using a solvent. The eluent is the solvent or mobile phase that passes through the column. When the polarity of the eluent matches the polarity of the molecules in the sample, the molecules desorb from the adsorbent and dissolve in the eluent.
The fraction of the mobile phase that transports the sample components is known as eluent. The mixture of solute and solvent that exits the column is known as an eluate. The eluate is made up of the mobile phase and analytes. A substance that separates and moves constituents of a mixture through the column of a chromatograph. The eluent in liquid chromatography is a liquid solvent whereas in gas chromatography is a carrier gas.
Column Chromatography Procedure
Before starting with the Column Chromatography Experiment let us understand the different phases involved.
Mobile phase – This phase is made up of solvents and it performs the following functions:
- It acts as a solvent-sample mixture that can be introduced in the column.
- It acts as a developing agent – helps in the separation of components in the sample to form bands.
- It acts as an eluting agent – the components that are separated during the experiment are removed from the column
- Some examples of solvents used as mobile phases based on their polarity are – ethanol, acetone, water, acetic acid, pyridine, etc.
Stationary phase – It is a solid material which should have good adsorption properties and meet the conditions given below:
- Shape and size of particle: Particles should have a uniform shape and size in the range of 60 – 200μ in diameter.
- Stability and inertness of particles: high mechanical stability and chemically inert. Also, no reaction with acids or bases or any other solvents was used during the experiment.
- It should be colourless, inexpensive and readily available.
- Should allow free flow of mobile phase
- It should be suitable for the separation of mixtures of various compounds.
Column Chromatography Experiment
- The stationary phase is made wet with the help of solvent as the upper level of the mobile phase and the stationary phase should match. The mobile phase or eluent is either solvent or a mixture of solvents. In the first step the compound mixture that needs to be separated, is added from the top of the column without disturbing the top level. The tap is turned on and the adsorption process on the surface of silica begins.
- Without disturbing the stationary phase solvent mixture is added slowly by touching the sides of the glass column. The solvent is added throughout the experiment as per the requirement.
- The tap is turned on to initiate the movement of compounds in the mixture. The movement is based on the polarity of molecules in the sample. The non-polar components move at a greater speed when compared to the polar components.
- For example, a compound mixture consists of three different compounds viz red, blue, green then their order based on polarity will be as follows blue>red>green
- As the polarity of the green compound is less, it will move first. When it arrives at the end of the column it is collected in a clean test tube. After this, the red compound is collected and at last blue compound is collected. All these are collected in separate test tubes.
Column Chromatography Applications
- Column Chromatography is used to isolate active ingredients.
- It is very helpful in separating compound mixtures.
- It is used to determine drug estimation from drug formulations.
- It is used to remove impurities.
- Used to isolate metabolites from biological fluids.
Types of Column Chromatography:
1. Adsorption column chromatography – Adsorption chromatography is a technique of separation, in which the components of the mixture are adsorbed on the surface of the adsorbent.
2. Partition column chromatography – The stationary phase, as well as mobile phase, are liquid in partition chromatography.
3. Gel column chromatography – In this method of chromatography, the separation takes place through a column packed with gel. The stationary phase is a solvent held in the gap of a solvent.
4. Ion exchange column chromatography – A chromatography technique in which the stationary phase is always ion exchange resin.
Frequently Asked Questions – FAQs
What is the principle involved in column chromatography?
The basic principle involved in column chromatography is to adsorb solutes of the solution with the help of a stationary phase and further separate the mixture into discrete components.
What is column chromatography?
It is a precursory technique used in the purification of compounds based on their hydrophobicity or polarity. In this chromatography process, the molecule mixture is separated depending on its differentials partitioning between a stationary phase and a mobile phase.
What is the main advantage of column chromatography?
The main advantage of this chromatography technique is that the stationary phase is less expensive and can be easily disposed of as it undergoes recycling.
How are the compounds separated in this technique?
The separation is similar to that of TLC where the compound mixture is carried by a mobile phase via a stationary phase.
Which compounds elute out first in the column chromatography technique?
Non-polar compounds. The polar compounds will strongly commune with the silica when compared to the non-polar compounds.
What is elution in column chromatography?
What are the limitations of column chromatography?
What are the different types of column chromatography?
What is an adsorption column chromatography?
What is gel column chromatography?
To learn more about the different types of Column Chromatography from the experts, register to BYJU’S now!
Other important links:
| Differential Extraction Chromatography | Difference between Adsorption and Absorption |

What is developing agent?
Chromatographic procedure generally involves introducing at the top of the column the mixture of the components to be separated, developing the mixture with a suitable agent, and collecting the components in separate effluent fractions. The best developing agent in thin-layer chromatography was Petroleum ether: ethyl acetate.