What is Cracking?
Cracking is defined as a process, wherein complex organic molecules namely long chain hydrocarbons or kerogens are broken down into smaller molecules namely light hydrocarbons. It is caused by the breaking of carbon-carbon bonds. The rate of catalyst greatly depends upon the factors such as the presence of catalyst and temperature. Cracking of hydrocarbons is illustrated in the below diagram.
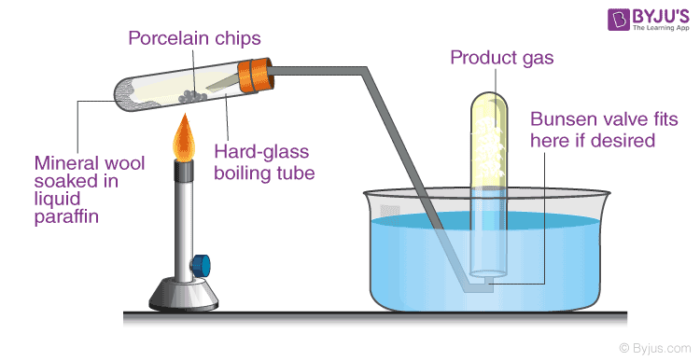
In petroleum refining, this process is used in the production of petrol, diesel, and gasoline. In chemistry, it is a process of breaking down large alkane into simpler alkenes and alkanes. Similarly, cracking of hydrocarbons includes breaking down of a complex long chain of hydrocarbons into smaller ones.
Cracking in Organic Chemistry
During this process, it involves numerous chemical reactions based on free radicals. Some vital reactions that take place are stated below.
Initiation: Here a single molecule breaks down into 2 free radicals. Only a smaller portion of freed radicals undergoes initiation, but it is sufficient to produce free radicals that are necessary to carry forward the entire reaction.
CH3CH3 → 2 CH3
Abstraction of Hydrogen: Here the second molecule becomes a free radical as it removes a hydrogen atom from another molecule.
CH3• + CH3CH3 → CH4 + CH3CH2•
Radical Decomposition: Here free radicals break into other free radical and an alkane. This reaction gives rise to alkene products.
CH3CH2• → CH2=CH2 + H
Radical Addition: This reaction results in the formation of aromatic products. Here radical reacts with an alkene to produce a free radical.
CH3CH2• + CH2=CH2 → CH3CH2CH2CH2•
Termination: Here 2 radicals react with each other to form products that are not free radicals. This reaction results in two forms namely recombination and disproportionation.
CH3• + CH3CH2• → CH3CH2CH3
CH3CH2• + CH3CH2• → CH2=CH2 + CH3CH3
Types of Cracking
- FCC: Fluid Catalytic Cracking: It is mainly used in petroleum refiners. This process involves the conversion of high molecular weight, high boiling hydrocarbons into olefinic, gases, gasoline and other products.
- Hydro cracking: It is a catalytic cracking process, where it uses hydro cracking to break C – C bonds. Products produced by this process include diesel, jet fuel, and LPG.
- Steam Cracking: It is a petrochemical process that involves the breakdown of saturated hydrocarbons into smaller unsaturated hydrocarbons.
- Thermal Cracking: It is a process that involves breaking of large non-volatile hydrocarbons into gasoline.
Frequently Asked Questions – FAQs
What is cracking organic chemistry?
Cracking is a reaction in which greater saturated hydrocarbon molecules are broken down into smaller, more functional hydrocarbon molecules, some of which are unsaturated: alkanes are the initial starting hydrocarbons. Cracking items contain alkanes and alkenes, part of a separate group of homologues.
What is cracking used for?
Cracking is a chemical process which is used in oil refining. To produce by-products such as cooking oil, ethanol, liquefied petroleum gas, diesel fuel, jet fuel and other petroleum distillates, cracking removes large hydrocarbon molecules in raw crude oil.
What happens during cracking?
Cracking is the mechanism of petrochemistry, petroleum geology, and organic chemistry whereby complicated organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons by breaking carbon bonds in the precursors.
Why is cracking so important?
For two key reasons, cracking is important: It helps balance the availability of fractions with the demand for them. When cracking transforms bigger hydrocarbons into smaller hydrocarbons, the fuel supply is increased. That helps to balance demand with supply.
What is needed for cracking?
Cracking is the term given for splitting up large clusters of hydrocarbons into smaller and more functional pieces. This is accomplished by using high pressures and temperatures in the presence of a catalyst, without a catalyst, or lower temperatures and pressures. It is only one way this particular molecule could break up.

I love these notes…I have really understand this aspect of chemistry
Verry fantastic notes