The chemical element iron has the atomic number 26 and the symbol Fe (from Latin: Ferrum). It’s a transition metal from group 8 of the periodic table’s first transition series. It is the most abundant element on Earth by mass, coming in second to oxygen (32.1% and 30.1%, respectively), and it makes up much of the planet’s outer and inner core. In the Earth’s crust, it is the fourth most abundant element.

Iron is a chemical element with an atomic number 26. A symbol Fe represents it. It is the most common element that is found on the earth. Unlike that of other elements, iron exists at oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment even though it is reactive to water and oxygen.
Iron is characterized by the ability to form variable oxidation states that differ in one or two organometallic chemistry. Since iron is available in abundance in nature, it is sometimes termed as a prototype for the entire block of a transition metal. Ferric is the iron (|||) compound, and ferrous is the iron (||) compound.
Compounds of iron are mainly formed at +2 and +3 oxidation states. They may also occur at higher oxidation state + 6. One of the excellent examples would be potassium ferrate. In a various biochemical oxidation reactions, iron (4) acts as an intermediate. Iron cannot reach an oxidation state of +8, and it is one of the first element of its group.
Electronic Configuration Of Iron
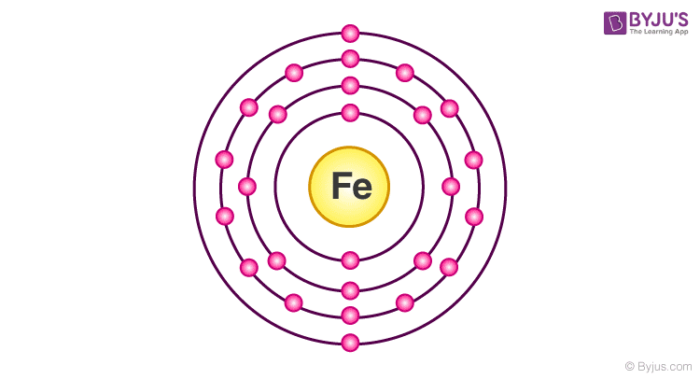
Electronic configuration of iron is [Ar] 3d64s2. Irons peculiar crystalline structure and electronic configuration make them naturally attractive to metals. It is termed as ferromagnetic materials. Iron exhibits different types of allotropic forms even though they do not contain a single crystalline structure. There are allotropic forms of iron and are termed as alpha, delta and gamma iron.
Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. The electronic configuration of Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Fe2+ contains 2 fewer electrons compared to the electronic configuration of Fe.
Applications of Iron
- Nitrates and iron chloride are used as industrial reagents. The iron sulfate is used in the fungicide.
- They are commonly used in the manufacturing of hulls of large ships, automobiles, various machine tools and machine parts.
- Iron Chloride used in treating sewage systems.
- Iron Sulphate is used to treat Iron Deficiency.
- Iron is nowadays used in various surgical equipment.
Frequently Asked Questions – FAQs
Why iron has 2 and 3 valency?
The energy of the 3d and 4s orbitals is nearly identical. It’s also worth noting that the 3d orbital has a single pair of electrons, whereas the others are unpaired. As previously stated, iron has two valence states of +3 and +2. As a result, it gains a valency of +2 when it gives away the two 4s electrons.
How Fe3O4 is formed?
Fe3O4 is made up of FeO and Fe2O3, or ferrous and ferric oxides, respectively.
Does iron have a full valence shell?
According to the electron configuration, the last shell of iron has two electrons, while the d-orbital has a total of six electrons. As a result, iron has eight valence electrons.
How many orbitals are required for the electronic configuration of iron?
We will put all 26 electrons in orbitals surrounding the nucleus of the Iron atom when we write the configuration. The initial two electrons in the electron configuration for Iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, Iron’s next two electrons are assigned to the 2s orbital.
How many bonds can iron form?
Iron might form six bonds, just like haemoglobin. Ionic compounds, on the other hand, do not share electrons; instead, they attract negative ions/atoms surrounding them.

Which electronic configuration corresponds to Fe in FeCl3?
Select one:
a. [Ar] 3d6
b. [Ar] 3d5
c. [Ar] 3d34s2
d. [Ar] 3d64s2
The electronic configuration corresponds to Fe in FeCl3 is [Ar]3d64s2.