What is Esterification?
The chemical reaction that takes place during the formation of the ester is called esterification.
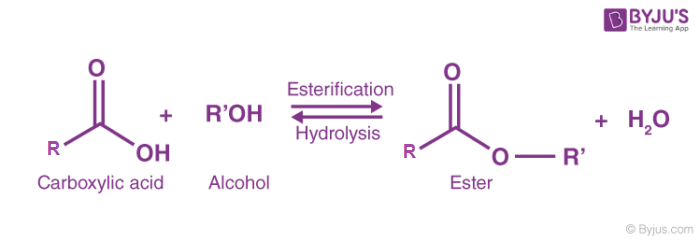
Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water; or a chemical reaction resulting in the formation of at least one ester product. Ester is obtained by an esterification reaction of an alcohol and a carboxylic acid.
Table of Contents
- What is Esterification Reaction?
- Esterification Mechanism
- Esterification Methods
- Properties of Esters
- Uses of Esters
- Recommended Videos
- Frequently Asked Questions – FAQs
The chemical reaction for esterification is given below.
What is Esterification Reaction?
When primary alcohol is treated with a carboxylic acid in the presence of sulphuric acid a compound is formed. This compound has a sweet smell. The compound obtained is called ester. The chemical reaction occurring in the formation of the ester is known as an esterification reaction.
CH3COOH + CH3CH2COOH → CH3COOCH2CH3
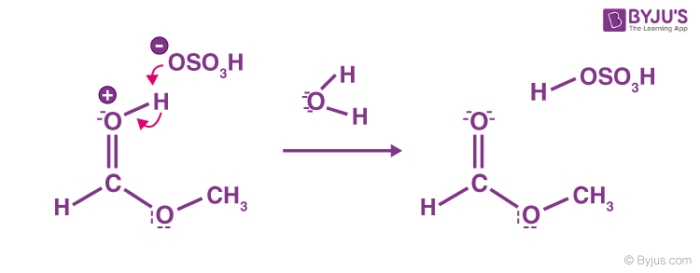
Esterification Mechanism
This process involves five steps. We have discussed the steps below:
Step 1: Cation formation
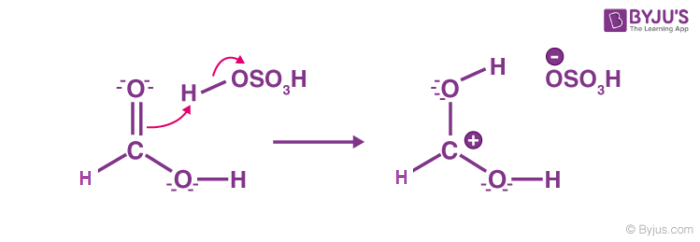
Step 2: Delocalized carbocation
Carboxyl oxygen gets protonated to give delocalized carbocation making the carbocation a better electrophile.
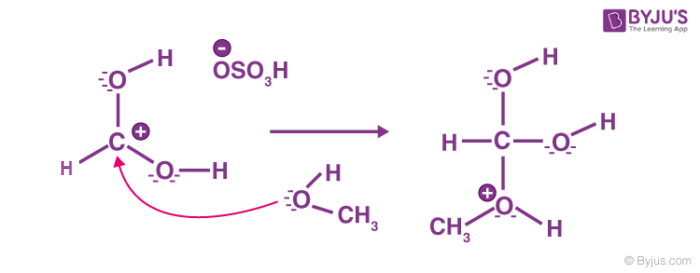
Step 3: Transfer of proton
A proton is transferred to one of the hydroxyl groups to form a good leaving group.
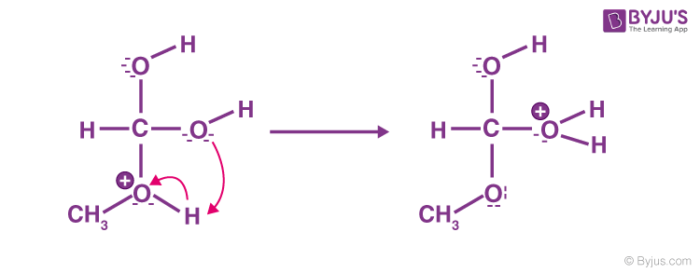
Step 4: Formation of the pi bond
The hydroxy group’s alcohol oxygen atom donates a pair of electrons to a carbon atom which makes a π bond by eliminating water. The concentration of water is less than methanol, therefore, it is not a feasible nucleophile to reverse the reaction.
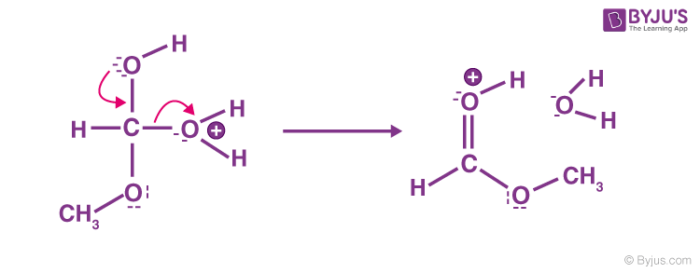
Step 5: Ester formation
Esterification Methods
Esterification can happen in three ways. They are discussed below:
- From acid anhydride and alcohol
- From acid chloride and alcohol
- From carboxylic acid and alcohol
1. Acid anhydride and alcohol
The reaction between alcohol and acid anhydride is comparatively slower when compared to acid chloride. To get a number of esters it is required to warm the mixture. For instance, consider 2,6-diiodophenol.
2,6-diiodophenol reacts with an acid anhydride to form ester.
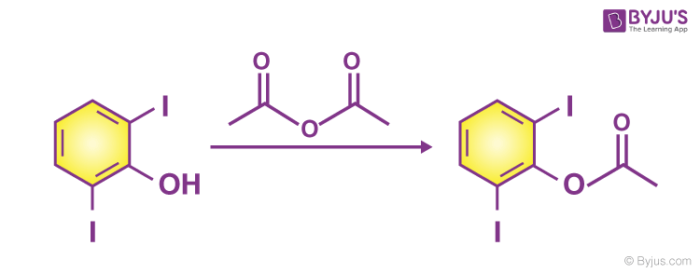
2. Acid chloride and alcohol
Esterification process can also be performed with alcohol and acid chloride at room temperature. Ester is obtained with steamy acidic fumes of hydrogen chloride. For instance, benzoyl chloride.
Reaction of alcohol and benzoyl chloride to form ester.
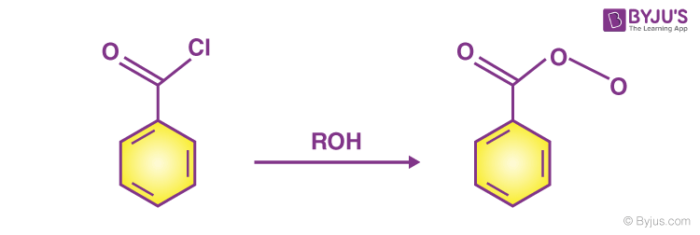
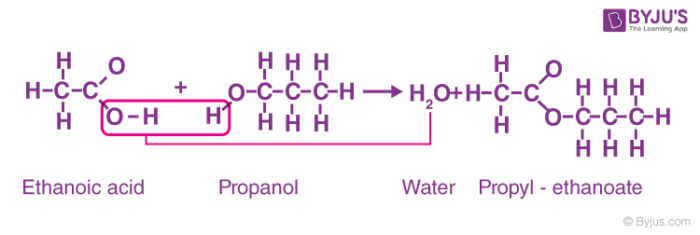
3. Carboxylic acid and alcohol
Production of esters from carboxylic acid and alcohol. Heat them in the presence of acid catalyst such as sulphuric acid (H2SO4) is used as a catalyst. For example, reaction of ethanoic acid and propanol to form propyl-ethanoate and water.
Properties of Esters
- The name of an ester is derived from its carboxylic acid that takes part in the esterification reaction.
- They have a pleasant smell.
- They have a wide application in the perfume and food industries.
- Esters are organic compounds found in oils and fats.
Uses of Esters
Some common uses of esters are mentioned below.
- Esters that have fragrance are used in perfumes, food flavourings, and cosmetics.
- Used as an organic solvent.
- Nitroglycerin is known and famous for its explosive properties.
- In the manufacturing of surfactants like detergents and soaps.
- Natural occurring esters are present in pheromones
- The backbone of DNA molecules is formed by phospo esters.
- Polyesters are used in the production of plastics
Recommended Videos
Frequently Asked Questions – FAQs
Give two examples of ester.
CH3COOCH3 and CH3CH2CH2CH2OH
Which compound has a higher boiling point: CH3COOCH3 or CH3CH2CH2CH2OH? Why?
CH3CH2CH2CH2OH due to the presence of intermolecular hydrogen bonding.
Give two main uses of the esterification reaction.
The two major uses of esterification reaction are:
1. Manufacturing of medicines.
2. Manufacturing of paints and dyes
What conditions are needed for esterification?
When a carboxylic acid reacts with an alcohol, esterification occurs. Only in the presence of an acid catalyst and heat can this reaction take place. Because it takes a lot of energy to remove the -OH from the carboxylic acid, a catalyst and heat are required to generate the required energy.
Why is saponification the reverse of esterification?
The esterification reaction is the inverse of the saponification reaction. In the presence of acid, a carboxylic acid and an alcohol react to form ester. In contrast, an ester reacts with a strong base or an acid to produce alcohol and carboxylic acid in the saponification reaction.

Comments