Etard Reaction Mechanism offers a way to oxidize aromatic methyl groups. Named to honour the French scientist Alexandre Leon Etard, the reaction details the oxidation of aromatic methyl groups or heterocyclic bound methyl groups to an aldehyde using chromyl chloride. For example, Toluene can be oxidized to Benzaldehyde using the Etard reaction as shown below:
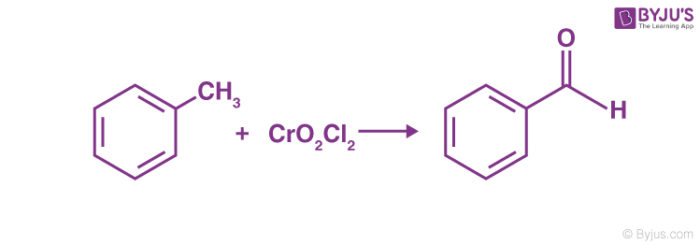
Etard Reaction Details
The reaction begins with an alkene – allylic hydrogen reaction with chromyl chloride, forming a precipitate called the Etard complex. This Etard complex is then decomposed with the help of a reducing environment to prevent its oxidation into a carboxylic acid. This reducing environment is generally provided by a saturated solution of aqueous sodium sulphite. Carbon tetrachloride is the most commonly used solvent for this method but carbon disulfide and chloroform can also be used as solvents. Aldehyde product of high purity can be obtained by purifying the Etard complex before its decomposition (so that its reaction with the unreacted reagent is prevented). The time frame for this equation may vary from a few days to several weeks but the yields are relatively high.
Etard Reaction Mechanism
An end reaction with chromyl chloride forming a precipitated Etard Complex is the first step of this mechanism.
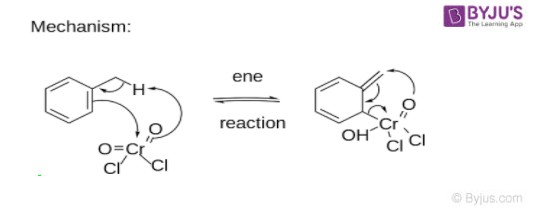
The precipitated Etard complex is decomposed (Purification of the precipitate before decomposition gives highly pure aldehyde product) by a [2,3] Sigmatropic Rearrangement (A pericyclic reaction where the end result is that one Sigma bond is changed to another sigma bond via an uncatalyzed intramolecular process). Reducing conditions provided by saturated aqueous sodium sulphite prevent further oxidation of the Etard complex into a carboxylic acid. Carbon tetrachloride is the most common solvent used in this process.
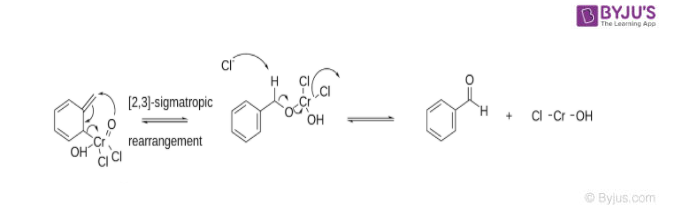
This reaction provides a very easy method for the oxidation of toluene to benzaldehyde which is utilized to add an almond flavour to foods and some cosmetic products, making it a valuable chemical. However, due to the sigmatropic rearrangement, obtaining aldehyde products from reagents other than toluene may prove difficult.


EXCELLENT