What is Glycine?
Glycine is one of the simplest kinds of amino acids occurring in nature. Amino acids are the essential components for all metabolic activities and life processes of human beings.
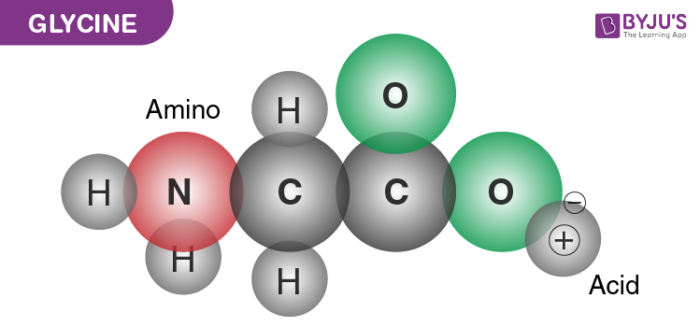
It is one among the list of 20 amino acids, which are important to human life. Glycine has a chemical formula C2H5NO2. It is the smallest of all the amino acids and consists of a side chain along with a hydrogen molecule as shown in the figure below.
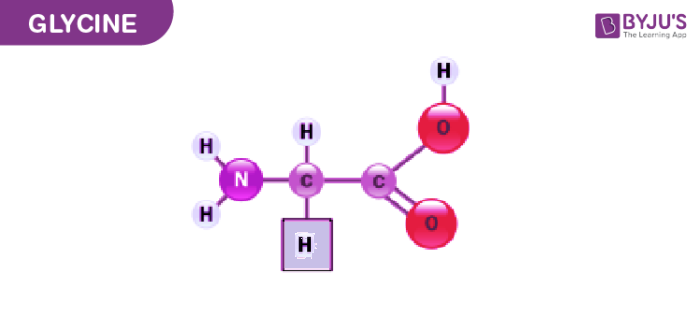
Glycine was isolated in the year 1820 from a substance called gelatin. Later it was discovered by a French chemist and pharmacist named Henri Braconnot. He obtained Glycine by boiling the gelatinous material with sulphuric acid.
Table of Contents
Properties of Glycine
Glycine exhibits various characteristic features as given below-
- It is highly soluble in water and is said to be a polar molecule.
- It appears as a colourless crystalline solid having a sweet taste.
- It is said to be hydrophilic in nature due to the minimal side chain having one hydrogen atom.
- This acts as a buffer solution at a pH of 6.00 due to its acidic nature
- It helps in the building up of proteins in the body and when mixed with carbohydrates, it improves lean growth and provides good recovery.
Glycine is one among the lists of non-essential amino acids present in mammals. They can also be synthesised from amino acids, such as threonine and serine and also from other sources. They do not require any kind of dietary sources.
Uses of Glycine
Glycine is a part of daily foods, such as protein-rich foods that include fish, meat, dairy products, legumes etc. Some of its uses are-
- It is used in the treatment of stroke, schizophrenia, benign prostatic hyperplasia along with some other rare inherited metabolic disorders.
- This crystalline solid is also used as a sweetener or a taste enhancer. Some food supplements and various protein-containing drinks have glycine.
- Certain formulations of the drug include glycine, which helps in improving gastric absorption.
- This acts as a buffering agent in analgesics, antacids, antiperspirants, cosmetics, toiletries, etc.
- It acts as an intermediate in the process of manufacturing different chemical products and is also used in manufacturing herbicide glyphosate.
Recommended Videos
Peptide Bonds

Frequently Asked Questions – FAQs
Why is glycine so important?
The simplest of amino acids, glycine is an integral component of crucial biological molecules, a central component of many metabolic reactions, a major neurotransmitter inhibitor in the spinal cord and brainstem, and an anti-inflammatory, cytoprotective and immune-modulating agent.
What is glycine made of?
Glycine is an organic compound which contains 2 atoms of carbon, 5 atoms of hydrogen, 1 atom of nitrogen and 2 atoms of oxygen. It is one of the 20 amino acids usually present in proteins found in animals. Glycine: an organic compound with the HO2CCH2NH2 formula. It is one of the 20 amino acids usually present in proteins found in animals.
What is the function of glycine?
A number of major metabolites such as glutathione, porphyrins, purines, haem, and creatine are precursors of glycine. In the central nervous system, glycine functions as a neurotransmitter and plays many roles in peripheral and nervous tissues, such as antioxidant, anti-inflammatory, cryoprotective and immunomodulatory.
Is glycine a weak acid?
Glycine’s activity is fairly representative of that of the simpler amino acids. Since glycine is neither a strong acid nor a strong base, four species in rapid equilibrium are required to produce glycine in water.
Define amino acids.
In simpler terms, Amino acids are organic compounds containing the basic amino groups (-NH2) and carboxyl groups (-COOH).
Continue reading about the Glycine through video lectures using BYJU’S the learning app.

All amino acids have optical activity except glycine ( Why)
Since glycerin has the same number of amine groups and acid groups, it is achiral. This is the reason why glycerin is optically inactive.