What is Halogenation Reaction?
A halogenation reaction is a chemical reaction between a substance and a halogen in which one or more halogen atoms are incorporated into molecules of the substance.
Table of Contents
- What is Halogenation of Alkanes?
- Recommended Videos
- General Reaction of Alkanes
- General Features of Halogenation of Alkanes
- Chlorination of Methane by Substitution
- Frequently Asked Questions on Halogenation of Alkanes
What is Halogenation of Alkanes?
Halogenation of an alkane produces a hydrocarbon derivative in which one or more halogen atoms have been substituted for hydrogen atoms.
Alkanes are notoriously unreactive compounds because they are non-polar and lack functional groups at which reactions can take place. Free radical halogenation therefore provides a method by which alkanes can be functionalized.
A severe limitation of radical halogenation however is the number of similar C-H bonds that are present in all but the simplest alkanes, so selective reactions are difficult to achieve.
Recommended Videos
Free Radical Mechanism of Halogenation

Saturated Hydrocarbons

General Reaction of Alkanes
Alkane halogenation is an example of a substitution reaction, a type of reaction that often occurs in organic chemistry. A substitution reaction is a chemical reaction in which part of a small reacting molecule replaces an atom or a group of atoms on a hydrocarbon or hydrocarbon derivative.
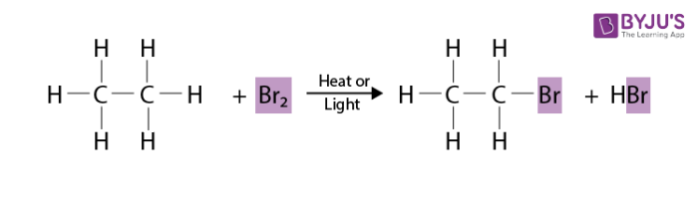
A general equation for the substitution of a single halogen atom for one of the hydrogen atom of an alkane is
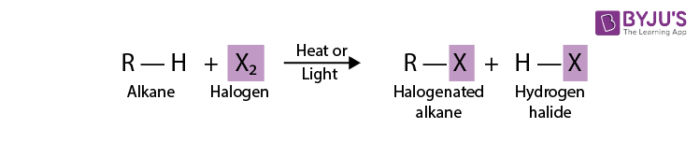
General Features of Halogenation of Alkanes
Note the following features of halogenation of alkanes.
- The notation R-H is a general formula for an alkane. R in this case represents an alkyl group. Addition of a hydrogen atom to an alkyl group produces the parent hydrocarbon of the alkyl group.
- The notation R-X on the product side is the general formula for a halogenated alkane. X is the general symbol for a halogen atom.
- Reaction conditions are noted by placing these conditions on the equation arrow that separates reactants from products. Halogenation of an alkane requires the presence of heat or light.
Chlorination of Methane by Substitution
In halogenation of an alkane, the alkane is said to undergo fluorination, chlorination, bromination or iodination depending on the identity of the halogen reactant. Chlorination and bromination are the two widely used alkane halogenation reactions. Fluorination reactions generally proceed too quickly to be useful and iodination reactions go too slowly.
Halogenations usually result in the formation of a mixture of products rather than a single product. More than one product results because more than one hydrogen atom on an alkane can be replaced with halogen atoms.
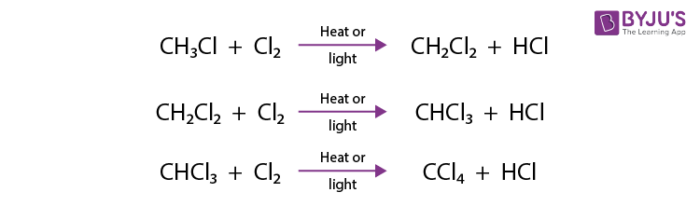
Methane and chlorine when heated to a high temperature in the presence of light react as follows.
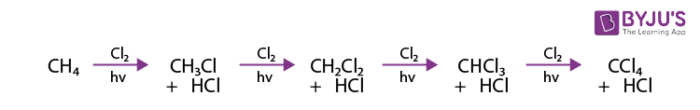
The mechanism for this reaction takes place in three steps.
1. Initiation Step:
The Cl-Cl bond of elemental chlorine undergoes hemolysis when irradiated with UV light, and this process yields two chlorine atoms, also called chlorine radicals.
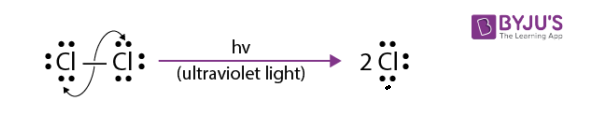
2. Propagation Step:
A chlorine radical abstracts a hydrogen atom from methane to produce the methyl radical. The methyl radical in turn abstracts a chlorine atom from a chlorine molecule and chloromethane is formed. The second step of propagation also regenerates a chlorine atom. These steps repeat many times until termination occurs.
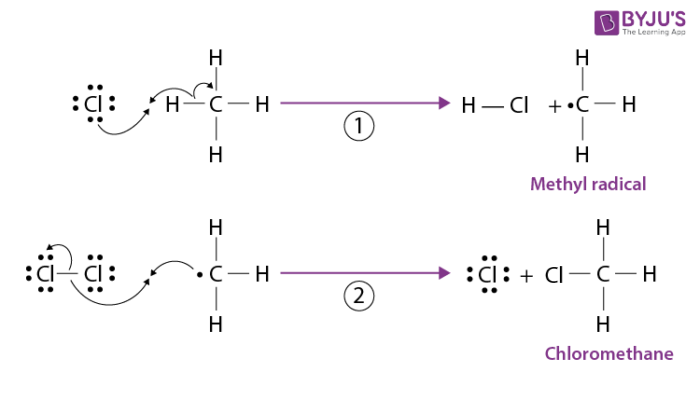
3. Termination Step:
Termination takes place when a chlorine atom reacts with another chlorine atom to generate Cl2, or chlorine atom can react with a methyl radical to form chloromethane which constitutes a minor pathway by which the product is made. Two methyl radicals can also combine to produce ethane, a very minor by product of this reaction.
The reaction does not stop at this step, however because the chlorinated methane product can react with additional chlorine to produce polychlorinated products.
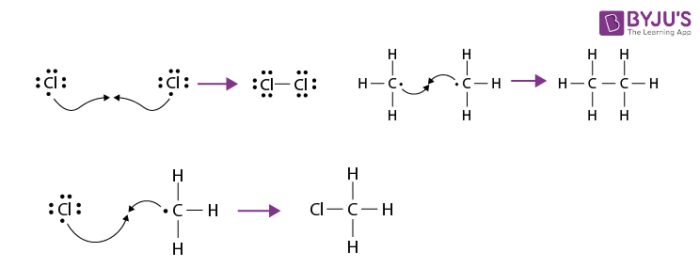
By controlling the reaction conditions and the ratio of chlorine to methane. It is possible to favour formation of one or another of the possible chlorinated methane products.
Halogenation via Free Radical Mechanism
Frequently Asked Questions on Halogenation of Alkanes
What is the halogenation reaction?
Halogenation is a reaction that occurs with the addition of one or more halogens to a substance. In the periodic table, Halogens form the seventh column and contain fluorine, chlorine, bromine, iodine and astatine. The substance resulting from a halogenation reaction is called a halogenated compound.
Is halogenation of alkanes a rearrangement reaction?
The halogenation regiochemistry of alkanes is generally determined by the relative weakness of the available C – H bonds. For the industrial processing of chlorinated methane, free radical halogenation is used: CH4 + Cl2 → CH3Cl + HCl. These free radical reactions also go with rearrangement.
Does halogenation of alkene need a catalyst?
Electrophiles attach to the double bond of alkenes that weaken the bond of pie. Contrary to alkene hydrogenation, catalysts do not allow the addition of molecular bromine or chlorine to generate nearby dichalcogenides.
What is the nature of the mechanism of halogenation of alkanes?
In the presence of ultraviolet (UV) light or heat, the reaction of a halogen with an alkane results in the formation of a haloalkane (alkyl halide). The phenomenon is explained by the reaction mechanism. Mechanism to halogenate. The carbon‐hydrogen bonds are low-polarity covalent bonds in the methane molecule.
What is the reaction of alkanes?
Alkanes (the most essential organic compound) are subjected to very few reactions. The two reactions of further imports are combustion and halogenation (i.e., substitution for a single halogen of a single hydrogen on the alkane).

Comments