What is Hypochlorous Acid?
HOCl is a weak acid with chemical name Hypochlorous Acid. It is also called Hydrogen hypochlorite or Chlorine hydroxide or hypochloric acid. It was discovered by a French chemist Antoine Jerome Balard in the year 1834. It is an oxyacid of chlorine.
It contains monovalent chlorine that functions as a reducing agent or an oxidizing agent. It is an unstable acid and functions as a human metabolite. It belongs to the family of reactive oxygen species and is a conjugate acid of a hypochlorite.
Properties of Hypochlorous Acid – HOCl
| HOCl | Hypochlorous Acid |
| Molecular weight of HOCl | 52.457 g/mol |
| No. of hydrogen bond acceptor | 1 |
| Monoisotopic mass of Hypochlorous Acid | 51.972 g/mol |
| No. of hydrogen bond donor | 1 |
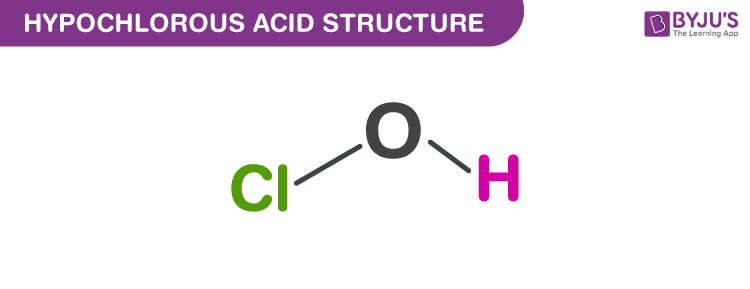
Hypochlorous Acid structure – HOCl
HOCl Uses (Hypochlorous Acid)
- Hypochlorous Acid is used to convert alkenes to chlorohydrins.
- Used in cosmetics such as baby products.
- Used in swimming pools.
- Used to generate sufficient quantities of safe disinfectant.
- Used in marine sanitation devices to convert seawater into HOCl.
- Used as a wound care agent.
- Used to treat various infections in pets and humans.
Reactions
-
- Hypochlorous acid partially dissociates into the anion hypochlorite ClO− in aqueous solutions. Below is the reaction:
HClO ⇌ ClO− + H+
Hypochlorites are the salts of hypochlorous acid. One of the common hypochlorites is sodium hypochlorite (NaClO), which is an active ingredient in bleach.
-
- At standard conditions Hypochlorous Acid is a stronger oxidant when compared to chlorine.
2 HClO(aq) + 2 H+ + 2 e− ⇌ Cl2(g) + 2 H2O
Where, E = +1.63 V
-
- Hypochlorous acid reacts with hydrochloric acid (HCl) to produce chlorine gas:
HClO + HCl → H2O + Cl2
-
- Hypochlorous acid reacts with amines to produce chloramines along with water.
NH3 + HClO → NH2Cl + H2O
Frequently Asked Questions – FAQs
What are the uses of hypochlorous acid?
What happens when acids are added to aqueous salts of hypochlorous acid?
How does hypochlorous acid react with amino acids?
Write the other name of hypochlorous acid.
Learn more about the Structure, physical and chemical properties of HOCl from the experts at BYJU’S.


Comments