What is Iron oxide?
Iron oxide, also called ferric oxide is an inorganic compound with the chemical formula Fe2O3.
It occurs in nature very abundantly and is widely distributed. It is a chemical complex which occurs naturally comprising iron and oxygen. Iron oxide is vital to humans and useful in most geological and biological activities. This iron oxide may be required for investigations of its own particular properties or used as starting materials for other processes.
Other names – Ferric oxide, Iron(III) oxide, Ferric oxide red
| Fe2O3 | Iron oxide |
| Density | 5.24 g/cm³ |
| Molecular Weight/ Molar Mass | 159.69 g/mol |
| Boiling Point | 3,414 °C |
| Melting Point | 1,565 °C |
| Chemical Formula | Fe2O3 |
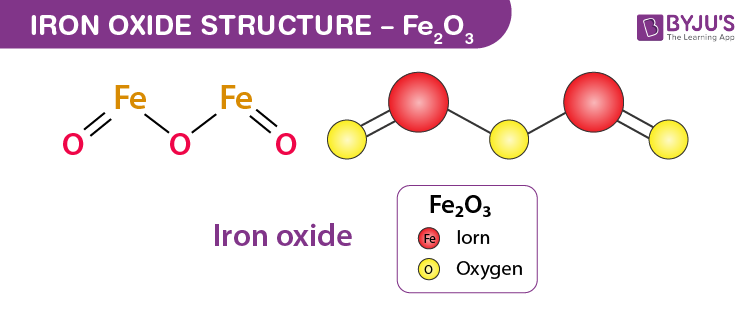
Iron oxide Structure – Fe2O3
Physical Properties of Iron oxide – Fe2O3
| Odour | Odourless |
| Appearance | Red-brown solid |
| Covalently-Bonded Unit | 5 |
| Hydrogen Bond Donor | 3 |
| Oxidation state | +3 |
| Solubility | Insoluble in water, soluble in strong acid |
Chemical Properties of Iron oxide – Fe2O3
-
- Iron oxide reacts with water to produce iron hydroxide.
Fe2O3 + H2O → Fe(OH)3
-
- Iron(III) oxide reacts with sulphuric acid to produce iron(III) sulfate and water.
Fe2O3 + H2SO4 → Fe2(SO4)3 + H2O
Uses of Iron oxide – Fe2O3
- The ordinary black iron oxide has been used in both copperplate and dye stamping inks.
- Oxides of iron constitute the main component of products in the pharmaceutical industry, paint industry, plastic industry, ink industry and cosmetic industry.
- Used as a pigment of natural origin including titanium dioxide.
- Its salt is used as a flocculant in wastewater treatment the dyeing of textiles and the production of fertilizer and feed additives.
- Used as a polishing material in the jewellery trade.
Frequently Asked Questions
Is iron oxide toxic to humans?
Iron oxide when breathed in will impact you. Exposure to fumes from Iron Oxide can cause fever from metal fumes. This is a flu-like condition with metallic taste signs, fever and chills, aches, chest tightness and cough. Ferrous Oxide (FeO), however, is highly flammable and reactive, and can spontaneously combust in air.
What is Fe3O4 called?
FeO is called ferrous oxide while Fe2O3 is ferric oxide. So the Fe3O4 compound is called ferrous ferric oxide.
What is the difference between Fe2O3 and Fe3O4?
They are ferrous oxides. Thus, Fe2O3 is a simple oxide where Fe is only + 3 in the oxidation state thus Fe3O4 is a mixed oxide where Fe is present in both + 2 and + 3 oxidation states. However, we compose Fe3O4 as FeO. Fe2O3 is written as iron oxide (III) while Fe3O4 is written as iron oxide (II, III).
What is iron oxide made of?
Iron oxides are compounds that are composed of iron and oxygen. There are seventeen known iron oxides and oxyhydroxides, of which the best known is rust, a type of iron oxide(III). Iron oxides and oxyhydroxides are common in nature and play a significant role in many processes, both geological and biological.
What is black iron oxide used for?
Black iron oxide or magnetite is used for resistance to corrosion, too. Often used in anti-corrosion paints is black iron oxide (found in many bridges, and Eiffel Tower). Iron oxides are used to shorten proton relaxation times (T1, T2 and T2) as a contrast agent in magnetic resonance imaging.


you are in reality a good webmaster. The web site loading velocity is incredible. It seems that you’re doing any distinctive trick. Furthermore, The contents are masterwork. you have done a excellent job in this matter!|