What is Isomerism?
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures.
Chemical compounds that have identical chemical formulae but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
The word “isomer” is derived from the Greek words “isos” and “meros”, which mean “equal parts”. This term was coined by the Swedish chemist Jacob Berzelius in the year 1830.
Table of Contents
- Isomerism Types
- Structural Isomerism
- Recommended Videos
- Stereoisomerism
- Ionization Isomerism
- Frequently Asked Questions – FAQs
Isomerism Types
There are two primary types of isomerism, which can be further categorized into different subtypes. These primary types are Structural Isomerism and Stereoisomerism. The classification of different types of isomers is illustrated below.
Structural Isomerism
Structural isomerism is commonly referred to as constitutional isomerism. The functional groups and the atoms in the molecules of these isomers are linked in different ways. Different structural isomers are assigned different IUPAC names since they may or may not contain the same functional group.
Recommended Videos
All About Structural, Geometrical & Optical Isomerism

Complete Isomerism in 6 Hours

Isomerism in Organic Compounds

The different types of structural isomerism are discussed in this subsection.
Chain Isomerism
- It is also known as skeletal isomerism.
- The components of these isomers display differently branched structures.
- Commonly, chain isomers differ in the branching of carbon
- An example of chain isomerism can be observed in the compound C5H12, as illustrated below.

Position Isomerism
- The positions of the functional groups or substituent atoms are different in position isomers.
- Typically, this isomerism involves the attachment of the functional groups to different carbon atoms in the carbon chain.
- An example of this type of isomerism can be observed in the compounds having the formula C3H7Cl.

Functional Isomerism
- It is also known as functional group isomerism.
- As the name suggests, it refers to the compounds that have the same chemical formula but different functional groups attached to them.
- An example of functional isomerism can be observed in the compound C3H6O.

Metamerism
- This type of isomerism arises due to the presence of different alkyl chains on each side of the functional group.
- It is a rare type of isomerism and is generally limited to molecules that contain a divalent atom (such as sulphur or oxygen), surrounded by alkyl groups.
- Example: C4H10O can be represented as ethoxyethane (C2H5OC2H5) and methoxy-propane (CH3OC3H7).
Tautomerism
- A tautomer of a compound refers to the isomer of the compound which only differs in the position of protons and electrons.
- Typically, the tautomers of a compound exist together in equilibrium and easily interchange.
- It occurs via an intramolecular proton transfer.
- An important example of this phenomenon is Keto-enol tautomerism.
Ring-Chain Isomerism
- In ring-chain isomerism, one of the isomers has an open-chain structure whereas the other has a ring structure.
- They generally contain a different number of pi bonds.
- A great example of this type of isomerism can be observed in C3H6. Propene and cyclopropane are the resulting isomers, as illustrated below.

Stereoisomerism
This type of isomerism arises in compounds having the same chemical formula but different orientations of the atoms belonging to the molecule in three-dimensional space. The compounds that exhibit stereoisomerism are often referred to as stereoisomers. This phenomenon can be further categorized into two subtypes. Both these subtypes are briefly described in this subsection.
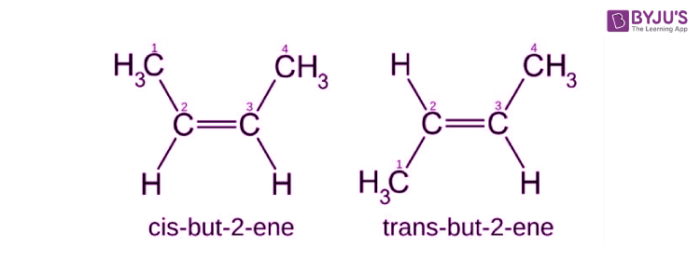
Geometric Isomerism
- It is popularly known as cis-trans isomerism.
- These isomers have different spatial arrangements of atoms in three-dimensional space.
- An illustration describing the geometric isomerism observed in the acyclic But-2-ene molecule is provided below.
Optical Isomerism
- Compounds that exhibit optical isomerism feature similar bonds but different spatial arrangements of atoms forming non-superimposable mirror images.
- These optical isomers are also known as enantiomers.
- Enantiomers differ from each other in their optical activities.
- Dextro enantiomers rotate the plane of polarized light to the right whereas laevo enantiomers rotate it to the left, as illustrated below.

Ionization Isomerism
The compound which gives different ions in the solution, although they have same composition, is called ionization isomers and this property is known as ionization isomerism. Compounds which gives different ions in solution although they have same composition are called ionization isomerism. This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion.
One example of ionisation isomerism is [Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4.
We can prepare these ionisation isomers in the following method.
[CoBr(NH3)5]SO4→ [CoBr(NH3)5]2+ + SO42− = Red−Violet[CoSO4(NH3)5]Br → [CoSO42−(NH3)5]+ + Br− = Red

Frequently Asked Questions – FAQs
What is isomerism in organic chemistry?
Isomerism in organic chemistry is a phenomenon shown by two or more organic compounds having the same molecular formula but different properties due to difference in arrangement of atoms along the carbon skeleton (structural isomerism) or in space (Stereo isomerism)
What is threo and erythro?
Erythro and threo are two configurations in which molecules are written when the molecules have a chiral carbon atom. Erythro is the configuration when the same groups are on the same side of the carbon atom and Threo is the configuration when the same groups are on the opposite side of the carbon atom.
What is Diastereoisomerism?
Diastereomers are defined as non-mirror image non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other.
What are the types of isomerism?
Two main forms of isomerism are structural or constitutional isomerism, in which bonds between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative positions of the atoms differ.
What are functional isomers?
Functional isomers are structural isomers that have the same molecular formula (that is, the same number of atoms of the same elements), but the atoms are connected in different ways so that the groupings are dissimilar. These groups of atoms are called functional groups, functionalities.
Thus, the concept of isomerism and its types are briefly discussed in this article. To learn more about this concept and other related concepts, such as the isotopes of an element, register with BYJU’S and download the mobile application on your smartphone.



Good notes by isomers
informative help a lot
Very good
This information is very correct and easy to understand
Nice
Tqq for providing a great information
Very good information about isomerism
Thank you for this great help
nice .thank you for providing a great information
Thank you for providing information
Thanks alot
good for refrences
thank u so much
thank you so much byjus !!! 😀😀😀
Thanks for giving such a useful information 🙏🙏
Thank you so much for this😊
This information really helped me thanks
Thank you 🙏it’s really helpful to me 👍
really very useful thank u soo much byjuse for providing this notes
Organic compounds having same molecular formula but differ from structure and arrange ment
Compounds that have the same molecular formula, but different chemical structures, are called isomers.
Really thank you so much 🙏byjus for providing this information