What is Magnesium hydroxide?
Magnesium Hydroxide is an inorganic compound which has a low solubility in water. It is also called Milk of magnesia or magnesium(2+) hydroxide.
Naturally, it occurs in the form of a mineral brucite and is a common compound found in antacids. The chemical formula of Magnesium hydroxide is Mg(OH)2.
Table of Contents
- History of Magnesium hydroxide
- Properties of Magnesium hydroxide – Mg(OH)2
- Properties of Magnesium hydroxide – Mg(OH)2
- Uses of Magnesium hydroxide (Mg(OH)2)
- Preparation of Magnesium hydroxide
- Effects on Health
- Frequently Asked Questions
History of Magnesium hydroxide
In the year 1872, Charles Henry Phillips was the first to use the term milk of magnesia. It was used for the suspension of magnesium hydroxide Mg(OH)2 formulated at about 8%w/v.
Properties of Magnesium hydroxide – Mg(OH)2
| Magnesium hydroxide | Mg(OH)2 |
| Molecular Weight of Magnesium hydroxide | 58.3197 g/mol |
| Density of Magnesium hydroxide | 2.3446 g/cm3 |
| Melting Point of Magnesium hydroxide | 350 °C |
| Monoisotopic Mass | 57.991 g/mol |
Structure of Magnesium hydroxide (Mg(OH)2)
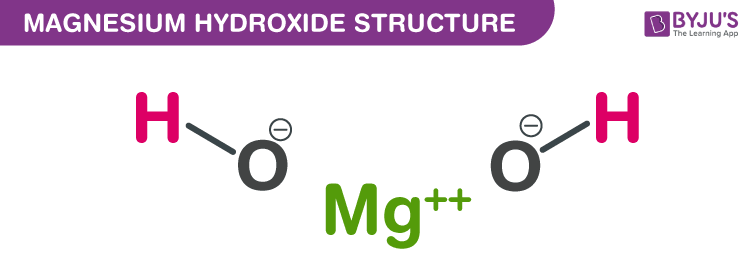
Structure of Magnesium hydroxide
Uses of Magnesium hydroxide (Mg(OH)2)
- Magnesium hydroxide is an excellent thermal conductor and poor electrical conductor.
- It is used in suspensions as a laxative or antacid.
- Used as a food additive.
- It is widely used in waste-water treatment.
- Used as a fire retardant.
- It is used in wet plate collodion process as a photographic fixer.
- It is used in gold mining.
- Used in warehouses.
Preparation of Magnesium hydroxide
When a solution of magnesium salts is combined with alkaline water it instigates precipitation of solid magnesium hydroxide. The reaction is as follows:
Mg2+ + 2 OH− → Mg(OH)2
Commercially it is produced by treating lime and seawater. Approximately one ton of magnesium hydroxide is obtained from 600 m3 of seawater. Calcium hydroxide is more soluble in magnesium hydroxide therefore Mg(OH)2 precipitates as a solid. The reaction is as follows:
Effects on Health
Flushing or drowsiness are side effects of magnesium hydroxide. Consuming this compound on a daily basis can result in electrolyte disturbances. Excessive use can result in nausea, diarrhoea, and abdominal cramping. Symptoms include watery diarrhoea or gastrointestinal irritation may occur. Poisoning causes hypomagnesemia which includes symptoms such as: vomiting, hypotension, confusion, muscle weakness, cardiac arrhythmias, Cardiac arrest, nausea, flushing, thirst, drowsiness, loss of tendon reflexes, respiratory depression, and coma.
Frequently Asked Questions – FAQs
What is magnesium hydroxide used for?
Magnesium hydroxide is used to soothe chronic constipation as a laxative. Magnesium hydroxide is also used as an antacid to treat indigestion, heartburn and acidic stomach
Is Milk of Magnesia the same as magnesium?
Magnesia milk can reduce constipation and improve heartburn and indigestion. Magnesium milk is sometimes referred to as magnesium hydroxide which is its chemical name. Magnesia milk is available to purchase without a prescription over the counter.
Is magnesium hydroxide a precipitate?
Mg(OH)2 is given partial precipitation with NH4OH by the magnesium ion (Mg2+). Mg(OH)2 is very sparsely soluble in water but is readily soluble in solutions of ammonium salts. Zirconium hydroxide is precipitated by the solutions NH4OH and NaOH.
What is the pH value of magnesium hydroxide?
The base is magnesium hydroxide. It has approximately a pH of 10. Magnesium hydroxide is usually called magnesium milk.
Is magnesium hydroxide a weak base?
Unlike lime, it is also much more difficult to treat magnesium hydroxide than it is caustic (NaOH). At neutral pH levels and higher, magnesium hydroxide is nearly insoluble in water. Keeping this in mind, mag has little to no effect on water alkalinity above 7.0 pH.

Comments