Introduction
Neutralisation is a chemical reaction where an acid and a base react with each other quantitatively. It is also written as Neutralisation. The acid strength of the reactant gives the pH of the neutralised solution.
Ever experienced a burning sensation in your stomach after eating too much spicy food? This is due to the formation of acid in the stomach. This problem can be cured by the consumption of an antacid which neutralizes the effect of acid, and this reaction is called a neutralisation reaction.
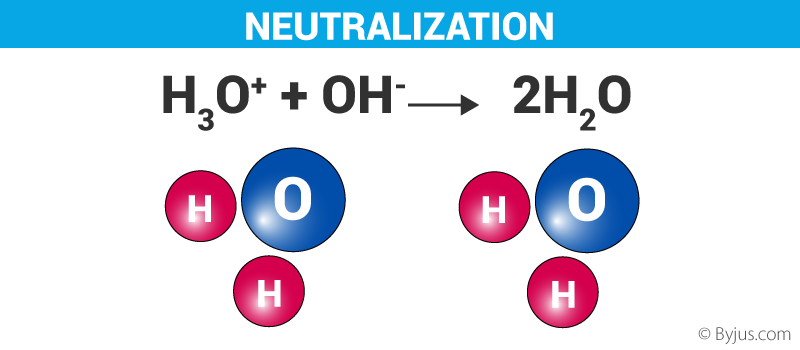
Table of Contents
- What is Neutralisation?
- Neutralisation Reaction
- Application of Neutralisation
- Frequently asked questions
What is Neutralisation?
It is an acid-base reaction in which an acid reacts with a base to form salt and water. The pH of the neutralised solution depends upon the acid strength of the reactants and their concentrations. The neutralisation reaction is best represented as:
Acid + Base → Salt + Water
Neutralisation Reaction
When a strong acid reacts with a strong base the resultant salt is neither acidic nor basic in nature i.e. it is neutral. For example when HCl (Hydrochloric acid), a strong acid, reacts with NaOH, a strong base, the resulting salt is sodium chloride and water.
HCl + NaOH → NaCl + H2O
When a strong acid reacts with a weak base the resultant salt is acidic in nature. For example, Fe(NO3)3 is an acidic salt formed due to the neutralisation of iron(III) hydroxide (a weak base) with nitric acid (strong acid)
3HNO3 + Fe(OH)3 → Fe(NO3)3 + 3H2O
Likewise when a strong base reacts with a weak acid then the resultant salt is basic in nature. For example, K2CO3 is formed due to the acid-base reaction of potassium hydroxide (strong base) and H2CO3 (weak acid).
H2CO3 + 2KOH → K2CO3 + 2H2O
When a weak acid and weak base react with each other complete neutralisation does not occur due to incomplete ionisation of the acid and base.
The most common strong acids and bases are mentioned in the table below.
Strong Acids |
Strong Bases |
|
|
Application of Neutralisation
- This method is used in wastewater treatment in order to reduce the damage created by the effluents.
- Neutralisation is used in the manufacturing of antacid tablets.
- The neutralisation reaction is used to control the pH of the soil.
Frequently asked questions
What is the pH value of strong acid and base during neutralisation?
The neutralisation of a strong acid and strong base has a pH value of 7.
How to neutralise NaOH with HCl?
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) + heat
Neutralisation reactions are irreversible. True or false?
False. Neutralisation reactions are reversible.
Give an example of neutralisation in day-to-day life.
Medicines such as anti-acids contain aluminium hydroxide and magnesium hydroxide to neutralise the excess acid in the stomach.
Briefly explain why the reaction between acid and base is termed a neutralisation reaction?
When acid and base react with each other, they form salt and water. Water and salt both are neutral which means, that whenever acid and base react together, they are neutralised by each other. Therefore, it is termed as a neutralisation reaction.
Read more:

For any further support on acids bases and salts, register with BYJU’S.

Comments