What is an Oxidizing Agent?
An oxidizing agent (often referred to as an oxidizer or an oxidant) is a chemical species that tends to oxidize other substances, i.e. cause an increase in the oxidation state of the substance by making it lose electrons. Common examples of oxidizing agents include halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H2O2).
Table of Contents
- Definition
- What Factors Affect the Oxidizing Power of an Oxidizing Agent?
- What are the Applications of Oxidizing Agents?
- Frequently Asked Questions – FAQs
Definition
Oxidizing agents can be defined in two different ways:
As an electron acceptor – They are chemical substances whose atoms remove at least one electron from another atom in a chemical reaction. As per this definition, oxidizing agents are the reactants that undergo reduction in redox reactions. An illustration detailing the electron-accepting properties of oxidizing agents is provided below.
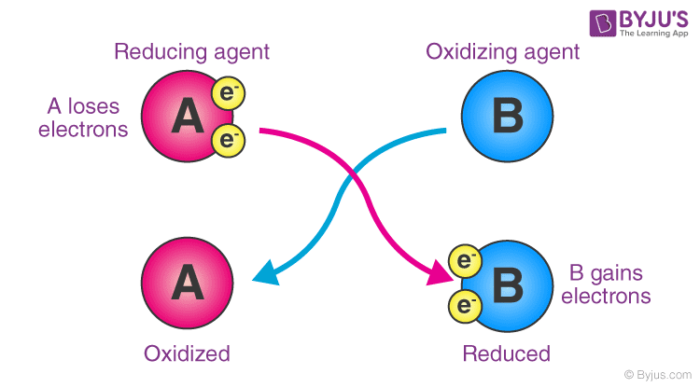
Here, substance ‘A’ undergoes oxidation, resulting in an increase in its oxidation number. On the other hand, the oxidation state of substance ‘B’ becomes smaller (since it gains electrons by undergoing reduction).
Click here to learn how to calculate oxidation numbers.
As an atom-transferring substance – An oxidizing agent is a substance that transfers at least one electronegative atom to a chemical species in a chemical reaction. The transferred atom is typically an oxygen atom. Several combustion reactions and organic redox reactions involve the transfer of an electronegative atom between two reactants.
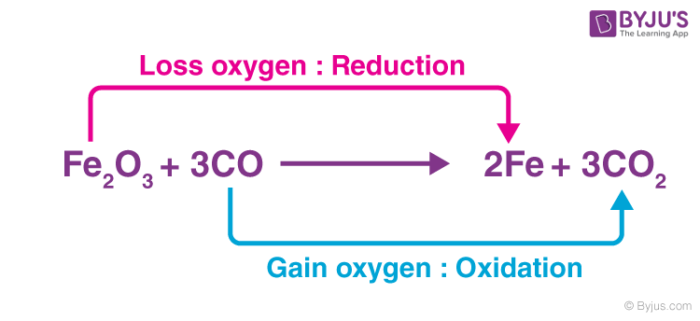
In the example illustrated above, the Fe2O3 molecule acts as an oxidizer by transferring an electronegative oxygen atom to the carbon monoxide molecule.
What Factors Affect the Oxidizing Power of an Oxidizing Agent?
Oxidizing agents normally exist in their highest possible oxidation states and, therefore, have a strong tendency to gain electrons and undergo reduction. Ions, Atoms, and molecules having a strong affinity towards electrons are considered to be good oxidizers. The stronger the electron affinity, the greater the oxidizing power.
Elemental fluorine is said to be the strongest elemental oxidizing agent. This is perhaps due to the fact that fluorine is the most electronegative element in the modern periodic table, and therefore exerts the strongest attractive force on electrons amongst all the elements. In fact, the oxidizing power of diatomic fluorine (F2) is strong enough to cause metals such as asbestos and quartz (and even molecules, such as water) to burst into flames when exposed to it.
A few other examples of elemental oxidizing agents include diatomic oxygen (O2), diatomic chlorine (Cl2), and ozone (O3). These oxidizers are the elemental forms of the second and the third most electronegative elements (oxygen and chlorine respectively), making them good electron acceptors.
The standard electrode potential of a half-reaction in a redox process provides insight into the oxidizing power of the chemical substance. An illustration ranking some oxidizers in terms of their oxidizing powers is provided below.
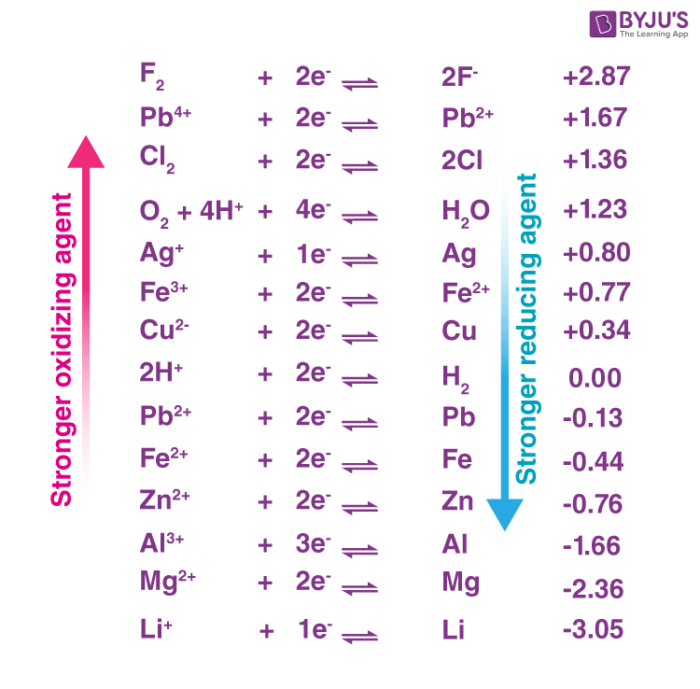
Some compounds that exhibit large oxidation states can also be considered good oxidizing agents. Ionic examples include the permanganate ion, the chromate ion, and the dichromate ion. Acidic examples of good oxidizers include nitric acid, perchloric acid, and sulphuric acid. The electronegativity of the molecules increases with the increase in the oxidation states of the atoms, increasing their ability to oxidize other substances.
Examples of Oxidizing Agents
Halogens
The group 17 elements of the periodic table are collectively referred to as Halogens. They are said to have a strong ability to gain electrons, attributed to their high electronegativities when compared to elements from other groups. This implies that they have the ability to easily attract electrons towards their respective nuclei. Examples of the halogens that are good oxidizing agents include iodine, bromine, chlorine, and fluorine. Fluorine is said to be the strongest elemental oxidizing agent due to its highest electronegativity, as discussed earlier.
Oxygen
Oxygen is the element corresponding to the atomic number 8 and is denoted by the symbol ‘O’. It belongs to the chalcogen group of the periodic table and is a highly reactive non- metal with good oxidizing properties. In general, metals tend to form metal oxides by reacting with atmospheric oxygen, due to the strong oxidizing power of oxygen. Oxygen is observed to be a part of a majority of combustion reactions.
Hydrogen Peroxide
Hydrogen peroxide is the chemical compound having formula H2O2. It appears to the human eye as a colourless liquid which has a greater viscosity than water. Hydrogen peroxide is the simplest compound having a peroxide functional group with an oxygen-oxygen single bond. It finds its uses as a weak oxidizing agent, disinfectant, and bleaching agent.
Many other oxidizing agents are commonly used industrially as well as in the day-to-day lives of humans. Examples include household bleach (NaClO), Potassium Nitrate (KNO3), and Sulphuric acid (H2SO4).
What are the Applications of Oxidizing Agents?
Oxidizing agents have numerous commercial and industrial applications. A few of these applications are listed below.
- Bleaching of fabrics.
- Purification of water.
- Combustion of fuel involves the use of an oxidizing agent.
- Storage of energy in batteries.
- Vulcanization of rubber (increasing the strength and the elasticity of rubber).
- Oxidizing agents are also vital to many biological processes such as metabolism and photosynthesis.
Many organisms make use of electron acceptors, or oxidizers, to collect energy from the redox reactions such as in the process of hydrolysis of glucose.
Recommended Videos
Mole Concept, Stoichiometry & Redox Reaction

Frequently Asked Questions on Oxidizing Agent
What distinguishes something as an oxidizing agent?
An oxidizing agent is a compound or element that participates in a redox (oxidation-reduction) reaction and accepts electrons from a different species. An oxidant is a chemical compound that easily transfers oxygen or another substance atoms in order to gain an electron.
Do oxidizing agents self-reduce?
By accepting electrons from other substances, oxidizing agents cause their oxidation states to become more positive. Oxidizing agents are reduced as well.
Does an oxidizing agent gains electrons?
Yes, Oxidizing agents gain electrons.
Why does oxidizing agent undergoes reduction?
When it comes to electron transfers, an oxidizing agent gains electrons while oxidation is the process of losing electrons. Reduction, on the other hand, means gaining electrons. As a result, the oxidizing agent gains electrons from the species being oxidized, implying that it undergoes reduction (as it gains electrons).
What is the difference between an oxidizing agent and a reducing agent?
An oxidizing agent is a substance that causes oxidation by accepting electrons and thus becoming reduced. A reducing agent is a substance that causes reduction by losing electrons, causing it to be oxidized.
To learn more about oxidizing agents and the part they play in oxidation-reduction reactions, register with BYJU’S and download the mobile application on your smartphone.

Amazing information and easily explained n understandable