What are Oxoacids Of Sulphur?
Oxoacids are acids that contain oxygen. Sulphur is known to form many oxoacids such as H2SO4, H2SO3, etc.
In oxoacids of sulphur, sulphur exhibits a tetrahedral structure when coordinated to oxygen.
Table of Contents
- Sulphuric acid
- Sulphurous acid
- Peroxodisulphuric acid
- Solved Example
- Frequently Asked Questions – FAQs
Generally, oxoacids of sulphur contain at least one S=O bond and one S-OH bond. Terminal peroxide groups, terminal S=S, terminal and bridging oxygen atoms and chains of (-S-)n are also observed in addition to S=S and S-OH.
Some popular oxoacids of sulphur and their properties are listed below:
Sulphuric acid, H2SO4
Sulphuric acid is one of the most popular oxoacids of sulphur. It is a diprotic acid (it ionizes to give two protons). In sulphuric acid, one atom of sulphur is bonded to two hydroxyl groups and the rest two oxygen atoms form pie bonds with the sulphur atom. Thus, sulphuric acid exhibits tetrahedral geometry.
As the bond length of sulphur oxygen bond (S=O) is smaller in comparison to the bond length of S-OH, OH groups are repelled by the oxygen atoms. Hence, the bond angle of O=S=O bond is larger than the HO-S-OH bond angle. Industrially, it is produced by the contact process.
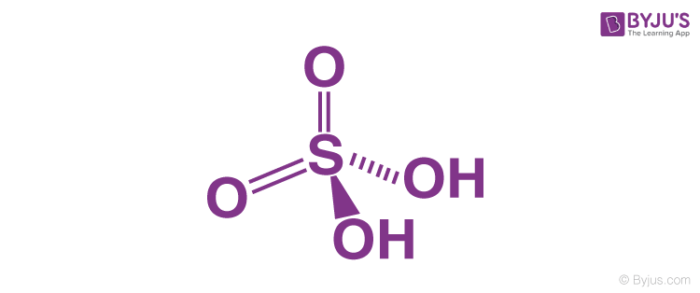
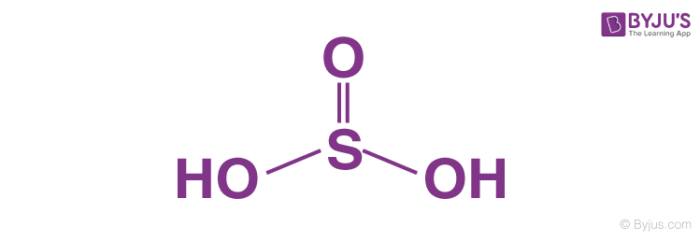
Sulphurous acid, H2SO3
Sulphurous acid is a diprotic acid that is, it ionizes two protons. In sulphurous acid, one atom of sulphur is bonded to two hydroxyl groups and one oxygen atom forms a pie bond with the sulphur atom. It is prepared by dissolving sulphur dioxide in water. Although, we don’t have any evidence of the existence of sulphurous acid in solution phase, the molecule can be isolated in the gaseous phase.
Peroxodisulphuric acid – H2S2O8
Peroxodisulphuric acid contains sulphur in +6 oxidation state. Thus, it is a strong oxidizing agent and highly explosive in nature. It is popularly known as Marshall’s acid. It contains one peroxide group forming a bridge between the two sulphur atoms. Each sulphur atom is connected to one hydroxyl group (S-OH bond) and two oxygen atoms (S=O bond)other than the peroxide group.
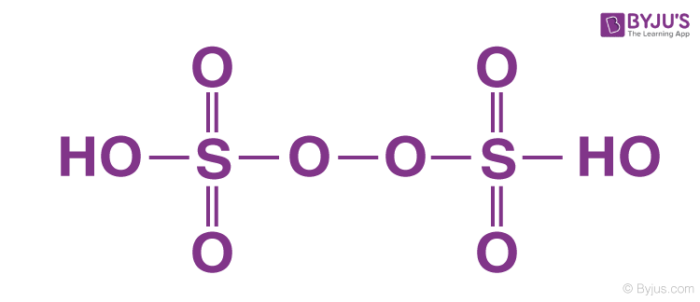
It is prepared by the reaction of the chloro sulphuric acid with hydrogen peroxide:
2ClSO3H + H2O2 → H2S2O8 + 2 HCl
Solved Example
Question:
In which of the following ions there is no S-S bond?
- S2O42−
- S2O62−
- S2O22−
- S2O72−
Solution:
S2O72− does not have S−S bond clearly mentioned by its structure.
Frequently Asked Questions – FAQs
Which are the Oxoacids of Sulphur?
The sulfur oxoacids are chemical compounds that contain sulfur, oxygen, and hydrogen. The best known and most important industrially used is sulfuric acid.
What are the 4 types of Oxyacids?
Chlorine forms four types of oxoacids. That is HOCl (hypochlorous acid), HOClO (chlorous acid), HOClO2(chloric acid) and lastly HOClO3 (perchloric acid).
Where sulphur is found?
Sulfur is found both in its native form and in metal sulfide ores. It occurs in its native form in the vicinity of volcanoes and hot springs. Sulfur is the 10th most abundant element, and it is found in meteorites, in the ocean, in the earth’s crust, in the atmosphere, and in practically all plant and animal life.
What are Oxyacids used for?
Nitric acid is heavily used in the laboratory and in chemical industries as a strong acid and as an oxidizing agent. The manufacture of explosives, dyes, plastics, and drugs makes extensive use of the acid. Nitrates are valuable as fertilizers.
What is Oxoacids chemistry?
An oxoacid (sometimes called an oxyacid) is an acid that contains oxygen. To be more specific, an oxoacid is an acid that: contains oxygen. contains at least one other element. has at least one hydrogen atom bonded to oxygen.
To learn more about the oxoacids of sulfur, register with BYJU’S and download our app.



Comments