There are different allotropic forms of phosphorus in nature. The important allotropic forms of phosphorus are white phosphorus, black phosphorus and red phosphorus. In this chapter, we will learn about the Phosphorus allotropic forms along with their properties.
Table of Contents
- White Phosphorus Properties
- Red Phosphorus Properties
- Black Phosphorus Pproperties
- Frequently Asked Questions – FAQs
- Translucent like white waxy solid
- Poisonous in nature
- Less stable and more reactive
- Does not dissolve in water
- When mixed with carbon disulphide it dissolves
- Glows in the dark(chemiluminescence)
- Further, the white form of it dissolves in NaOH in an inert atmosphere giving PH3. The reaction that takes place is as follows:
- \(\begin{array}{l} P_4 + 3NaOH + 3H_20 \rightarrow PH_3 + 3NaH_2PO_2\end{array} \)
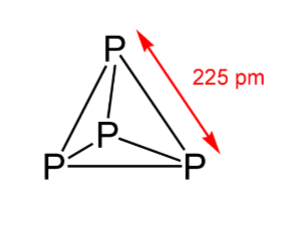
- Due to the angular strain in P4 molecule (wherein the angle is 60∘), In the presence of air, it readily catches fire and gives out dense white fumes of P4O10. The reaction is as follows: P4+5O2→P4O10
- The below figure shows that it has a discrete tetrahedral P4 molecule:
- On heating white P4 at 573K in an inert atmosphere for several days, red phosphorus is obtained. Further, on heating it under high pressure, a series of phases of black form is formed.
- Has iron grey lustre
- Odourless
- Non-poisonous
- Solubility properties: does not dissolve in water and carbon disulphide
- Does not glow in the dark
- Chemically, white phosphorus is more reactive than red phosphorus
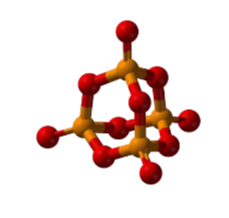
- It is polymeric and consists of chains of P4 tetrahedra linked together as shown in the figure given below
White Phosphorus Properties:
(Sodium Hypophosphite)
Red Phosphorus Properties:
Black Phosphorus properties:
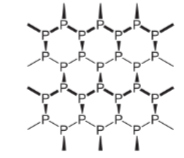
α-black and β-black are two forms of black phosphorus. When red phosphorus is heated in a sealed tube at 803K, an α-black form is formed. It can be sublimed in the air and has either rhombohedral crystals or opaque monoclinic. It does not oxidize in the air. When white form is heated under high pressure at 473 K β-Black is formed. It does not burn in the air up to 673K. Structure of the black form of this substance is shown below:
Frequently Asked Questions – FAQs
Why is phosphorus p4?
Since it can form three bonds, phosphorus can form a P4 white phosphorus tetrahedron, while sulphur can only form two bonds. Hence, sulphur only forms rings and chains. A cross-linked, polymeric chain of atoms is the most soluble allotrope of phosphorus, red phosphorus.
Which phosphorus is used in matchstick?
A small amount of the red phosphorus on the hitting surface is converted into white phosphorus as the match is lit, which then ignites. The potassium chlorate is ignited by the heat from this, and the match head explodes into flame.
Which allotrope of phosphorus is poisonous?
The least stable, the most reactive, the most volatile, the least dense, and the most toxic of the allotropes is white phosphorus. It eventually changes to red phosphorus, a light and heat-accelerated transition.
Why is phosphorus called the devil’s element?
Owing to its eerie glow, ability to burst into fire, and since it was the 13th known chemical, some texts refer to phosphorus as the “Devil’s Chemical.” Pure phosphorus, like other non-metals, assumes substantially different shapes. At least five phosphorus allotropes exist.
What is special about phosphorus?
Phosphorus is a vital plant nutrient and its primary application is in fertiliser production by phosphate compounds. Just when there are cycles of biological carbon and nitrogen, there is a phosphorus cycle as well. In making protective matches (red phosphorus), pyrotechnics and explosive bombs, phosphorus is included.
Visit BYJU’S, India’s best Edu-tech company to see the magic that is created when education meets technology.

way of representation is nice !!!!!!!!