What is Potassium Hydroxide?
Potassium hydroxide is an inorganic compound which is denoted by the chemical formula KOH.
Potassium hydroxide is also known as caustic potash, lye, and potash lye. This alkali metal hydroxide is a very powerful base. The aqueous form of potassium hydroxide appears as a clear solution. In its solid form, KOH can exist as white to slightly yellow lumps, flakes, pellets, or rods. No characteristic odour can be attributed to this compound in its solid state.
Table of Contents
- Properties of Potassium Hydroxide
- Potassium hydroxide Structure
- Uses of Potassium Hydroxide
- Chemical Reactions Undergone by KOH
- Health Hazards of KOH
- Frequently Asked Questions – FAQs
Potassium hydroxide is soluble in water, freely soluble in ethanol, methanol, and glycerin. It is slightly soluble in ether. It is non-combustible but highly corrosive. It is widely used in chemical manufacturing, cleaning compounds, and petroleum refining.
Properties of Potassium Hydroxide – KOH
| KOH | Potassium hydroxide |
| Molecular weight/molar mass of KOH | 56.11 g/mol |
| Density of Potassium hydroxide | 2.12 g/cm3 |
| Boiling Point of Potassium hydroxide | 1,327 °C |
| Melting Point of Potassium hydroxide | 360 °C |
Potassium hydroxide Structure – KOH
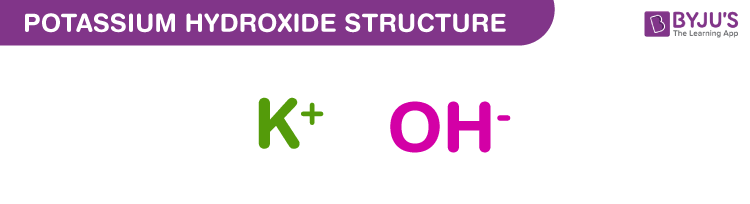
Potassium hydroxide structure
Uses of Potassium Hydroxide
- Potassium hydroxide solution is more conductive when compared to NaOH and therefore used as an electrolyte in some alkaline batteries.
- It is used as a pH control agent in the food industry.
- It is used in the thickening of food.
- It is used in chip fabrication for semiconductors.
- It is used in the manufacturing of cuticle removers which are used in manicure treatment.
- It is used in the identification of species of fungi.
- It is used in mercerizing cotton.
- It is used in alkalimetric titrations in analytical chemistry.
- Used in the manufacturing of liquid fertilisers.
Chemical Reactions Undergone by KOH
1. Saponification of ester
The ester is saponified by heating with a known amount of potassium hydroxide in an organic solvent in a sealed tube. To be useful analytically, this reaction must be quantitative in a reasonable length of time. One condition that favours a rapid and quantitative reaction is the use of KOH as a strong base as possible.
KOH + RCOOR’ → RCOOK + R’OH
2. KOH reacts with CO2 to produce bicarbonate
The addition of hydroxide ions by adding lime, sodium hydroxide, or potassium hydroxide, adjusts the pH because the hydroxide ion reacts with carbon dioxide to form bicarbonate alkalinity.
KOH + CO2 → KHCO3
Health Hazards of KOH
The health hazards of potassium hydroxide are similar to those of the other strong alkalies, such as sodium hydroxide. Potash lye and its solution can severely irritate skin, mucous membranes, and eyes. When it comes in contact with water or moisture it can generate heat to instigate combustion. Potassium hydroxide is corrosive to tissues.
Frequently Asked Questions – FAQs
What is potassium hydroxide used for?
Potassium hydroxide, or caustic potash, is used in a wide variety of industries. It is used in the chemical industry, mining, manufacturing of different compounds, fertilisers, in potassium soaps and in detergents.
What are the dangers of potassium hydroxide?
Causes eye pain, tearing, redness and swelling. Larger exposures cause serious burns with potential subsequent blindness. Chronic exposure: repeated contact with dilute solutions of potassium hydroxide dust has a tissue-destroying effect.
Is potassium hydroxide a carcinogen?
The National Toxicology Program (NTP), the International Agency for Research on Cancer (IARC), and the Occupational Safety and Health Administration (OSHA) do not recognize potassium hydroxide as a carcinogen. Potassium hydroxide is of low toxicity to marine species.
What is potassium hydroxide in chemistry?
Potassium hydroxide, also called lye, is an inorganic compound containing the chemical formula KOH. Often commonly called caustic potash, it is a strong base that is sold in different forms including pellets, flakes, and powders.
What is the pH of KOH?
KOH is an example of a strong base which means that it dissociates completely in an aqueous solution into its ions. Although the pH of KOH or potassium hydroxide is extremely high (typical solutions typically range from 10 to 13), the exact value depends on the concentration of this strong base in water.
Learn more about the Structure, physical and chemical properties of KOH from the experts at BYJU’S.

Comments