Soaps are an integral part to maintain the good health and hygiene of individuals. Soaps are essential to cleanse dirt and oil off the objects including the skin surface. Soaps are widely used in bathing, cleaning, washing and in other household chores.
Table of Content
Saponification Definition
Saponification is the hydrolysis of an ester with NaOH or KOH to give alcohol and sodium or potassium salt of the acid.
Soap is now an essential everyday item and finds its importance in everyday life. But, how is soap made? The process of making soap is called saponification. Here, the soap making process or saponification is discussed in a detailed and easy way.

What is Saponification?
Saponification is simply the process of making soaps. Soaps are just potassium or sodium salts of long-chain fatty acids. During saponification, ester reacts with an inorganic base to produce alcohol and soap.
Generally, it occurs when triglycerides are reacted with potassium or sodium hydroxide (lye) to produce glycerol and fatty acid salt, called ‘soap’.
Related Videos on Saponification
Saponification Reactions
Triglycerides are generally animal fats and vegetable oils. When they are reacted with sodium hydroxide, a hard form of soap is created. This is where potassium comes in and creates a softer version of the soap.
The equation can be written as:
| Ester + Base ————–> Alcohol + Soap |
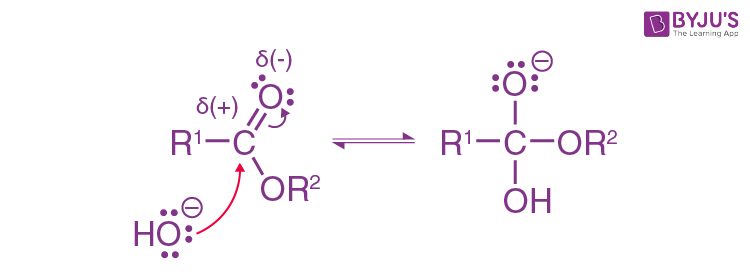
Saponification Reaction Mechanism
Orthoester formation:
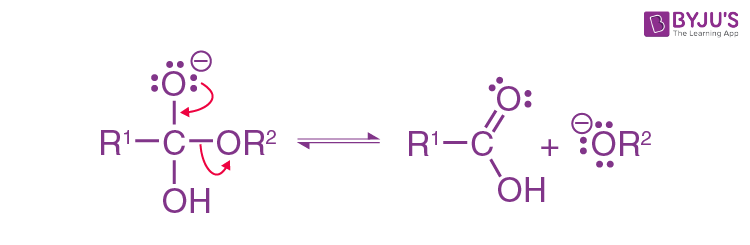
 Expulsion of carboxylic acid and alkoxide:
Expulsion of carboxylic acid and alkoxide:
 Formation of alcohol:
Formation of alcohol:
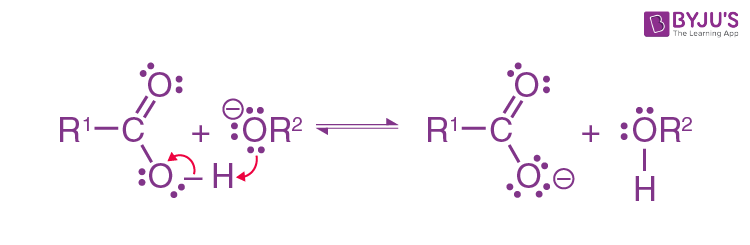
Examples of a Saponification Reaction:
In a saponification reaction, a base (for example sodium hydroxide) reacts with any fat to form glycerol and soap molecules. One of the saponification reactions taking triglyceride as an ester and sodium hydroxide as the base is as follows:
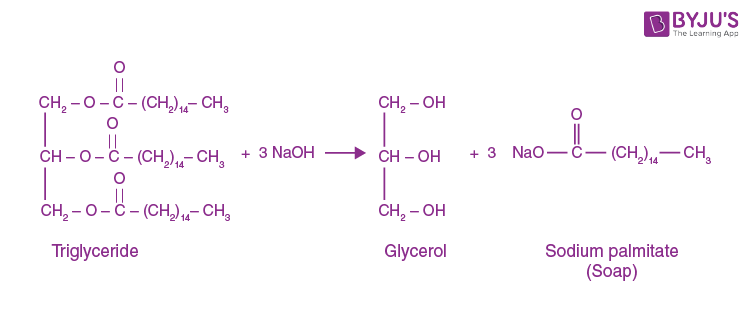
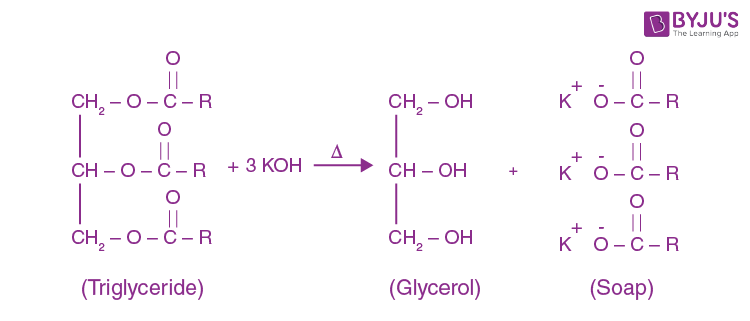
1-Step Saponification vs 2-Step Saponification
There can be either one-step saponification or two-step saponification process to convert triglycerides to soaps. The examples mentioned above are a one-step saponification process in which triglycerides, when treated with a strong base, split from the ester bond to release glycerol and soaps (i.e. fatty acid salts).
On the other hand, in the two-step saponification process, the steam hydrolysis of the triglyceride yields glycerol and carboxylic acid (rather than its salt). In the second step, alkali neutralizes fatty acids to produce soap.
Saponification Value
Saponification value or saponification number refers to the amount of base that is required to saponify a fat sample. Generally, saponification values are listed in KOH and so, saponification value can also be defined as that value which represents the number of milligrams required to saponify 1 gram of fat under the specified conditions.
In case sodium hydroxide is used for the saponification process, the saponification value must be converted from potassium to sodium by dividing the KOH values by the ratio of the molecular weights of KOH and NaOH (i.e. 1.403).
Effects of Saponification
The effects of saponification can either be desirable or undesirable. Some effects of saponification are mentioned in the below-given points.
- One of the major desirable effects of saponification is seen in fire extinguishers. Saponification is used by wet chemical fire extinguishers to convert burning fats and oils into non-combustible soap which helps in extinguishing the fire. Further, the reaction is endothermic and lowers the temperature of the flames by absorbing heat from the surroundings.
- In an undesirable scenario, saponification damages oil paintings. In oil paintings, the heavy metals used in pigments react with the oil containing free fatty acids and form soaps. This way, the paintings get damaged gradually.
- Soaps formed are used in everyday life like sodium soaps are used for laundry, potassium soaps are used for cleaning and lithium soaps are used as lubricating greases. There are various other soaps which are used for different purposes.
Uses of Saponification
- Wet chemical fire extinguishers: To extinguish cooking oils and fats, we use a saponification reaction. This is because cooking oils and fats have a flashpoint which is above 37 degrees which renders regular fire extinguishers useless.
- Creating hard and soft soaps: By using different types of alkali in the process the type of reaction product can be altered between hard and soft.
- Using KOH: We can obtain soft soaps
- Using NaOH: We can obtain hard soaps
This was all about the saponification process. This topic is one of the most crucial topics in chemistry and students are required to be thorough with the reactions and terms to be able to answer questions in the exam.
| Also, Read: Soap and detergent – Warriors Against Germs |
Frequently Asked Questions – FAQs
What is saponification and its reaction?
Ester reacts with an inorganic base during saponification to create alcohol and soap. It normally happens as potassium or sodium hydroxide (lye) reacts to triglycerides to create glycerol and fatty acid salt, called ‘soap’.
What is a saponification example?
Saponification is the hydrolysis of an ester to form an alcohol and the salt of a carboxylic acid in acidic or essential conditions. Saponification is usually used to refer to the soap-forming reaction of a metallic alkali (base) with fat or grease. Example: In the presence of conc., ethanoic acid reacts with alcohol.
What is the definition of saponification in science?
Saponification can be characterized as a “hydration reaction in which free hydroxide breaks the bonds of ester between triglyceride fatty acids and glycerol, resulting in free fatty acids and glycerol,” each of which is soluble in aqueous solutions.
Why is saponification important?
Saponification is the hydrolysis of fats or oils for the extraction of glycerol and the salt of the resulting fatty acid under simple conditions. Knowing the amount of free fatty acid present is essential to the industrial consumer, as this determines the processing loss to a large degree.
What type of reaction is saponification?
Saponification is a type of chemical reaction in which ester molecules are broken to create a functional group of carboxylic acid and alcohol. A collection of molecules or atoms that we can readily recognize in a compound is a functional group. To produce soap goods, this reaction is most widely used.
To learn more important chemistry topics in an easy and effective way, stay tuned with BYJU’S. At BYJU’S, students are provided with easy explanations of different topics, sample papers, question papers and effective preparation strategies to aid in chemistry preparation.

very useful and outstanding information is given by you
i am really very interested by studying with you
i advise all of you to be connect with byjus
Very useful especially in this quarantine time