Methods of Preparation of Aldehydes
The organic compounds that contain carbon-oxygen double bonds are referred to as carbonyl compounds. The Carbonyl group is one of the most significant functional groups in organic chemistry. Some of these compounds are widely used in the industry for manufacturing various chemicals and reagents.
Carbonyl compounds are of two types, aldehydes and ketones. The compounds in which the carbonyl group is attached to carbon and hydrogen are called aldehydes, while the compounds in which the carbon group is attached to two carbon atoms are called ketones. The aldehydic or aldehyde group is denoted as -CHO, whereas the ketone or ketonic group is denoted using -CO. This article is concerned with the methods of preparation of aldehydes.
Recommended Videos

Preparation of Aldehydes
There are various methods that can be used to prepare aldehydes depending upon the type and requirement of the compounds. Following are some important methods of preparation of aldehydes.
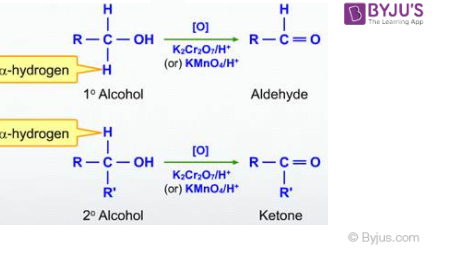
1. By Oxidation of Alcohol
After the oxidation of primary and secondary alcohols, we can get both aldehydes and ketones. The oxidation of primary alcohols results in the formation of aldehydes while the oxidation of secondary alcohols produces ketones. The general chemical reaction for oxidation of primary and secondary alcohol is shown as:
2. Dehydrogenation of Alcohols
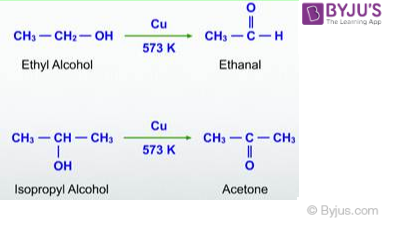
Dehydrogenation means removal of hydrogen. Dehydrogenation of alcohols is widely used in industries. In this method, a primary alcohol is passed over metal catalysts like Copper which results in the formation of an aldehyde. This method is preferred for the conversion of volatile alcohols into aldehydes. For example, the reaction of ethyl alcohol and isopropyl alcohol with Cu as a catalyst at 573 K temperature produces ethanal and acetone respectively.
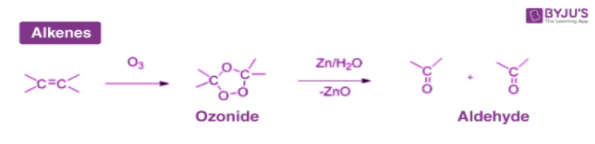
3. From Hydrocarbons
Aldehydes are obtained by the ozonolysis of alkenes.
In ozonolysis, first, an alkene reacts with an ozone molecule which results in the formation of ozonide. Then the ozonide so formed reacts with zinc dust and water which results in the formation of either aldehyde or ketone depending on the type of hydrocarbon used.
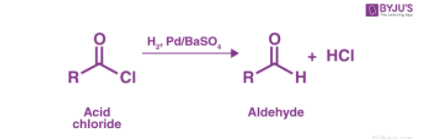
There are other methods used for the preparation of aldehydes, let’s have a look at those methods:
- From acyl chloride: The hydrogenation of acid chlorides by passing over a catalyst such as palladium aldehydes is obtained. This reaction is called Rosenmund reduction.
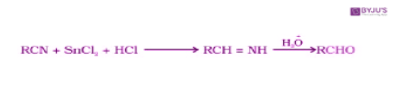
- From nitriles and esters: The reaction of nitriles with stannous chloride and hydrochloric acid produces imine first. then the imine so formed undergoes hydrolysis to produce aldehyde as the product. This reaction is called the Stephen Reaction
Click here to know about the Nomenclature of Aldehydes.
We can get aromatic aldehydes with the help of aromatic hydrocarbons by using the methods given below:
4. By Oxidation of Methylbenzene
Toluene can be oxidised by strong oxidising agents to benzoic acid. The oxidation can be stopped at the aldehyde stage by using those suitable reagents to convert the methyl group to an intermediate that cannot be oxidised further.
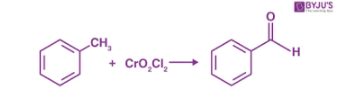
- By using chromyl chloride(CrO2Cl2): The reaction of toluene with chromyl chloride results in the formation of a chromium complex which on further hydrolysis produces benzaldehyde. This reaction is called the Etard reaction.
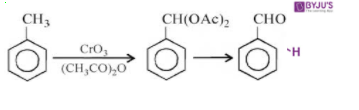
- By using chromic oxide(CrO3): The reaction of toluene or substituted toluene in the presence of chromic acid in acetic anhydride produces benzylidene diacetate. Then the benzylidene diacetate on hydrolysis gives the corresponding benzaldehyde.
- By Gatterman –Koch reaction: The treatment of carbon monoxide and hydrogen chloride with benzene or its derivative in the presence of aluminium chloride gives benzaldehyde or substituted benzaldehyde. This is called Gatterman –Koch reaction.

Frequently Asked Questions – FAQs
What is meant by aldehyde?
Aldehyde, part of a family of organic compounds in which a carbon atom forms a double bond with an oxygen atom, one single bond with a hydrogen atom, and one single bond with another atom or group of atoms known as R in general chemical formulas and structural diagrams.
Is aldehyde acidic or basic?
Aldehydes have a sp2-hybridised, planar carbon core that is bound to oxygen by a double bond and hydrogen by a single bond. Typically, the C – H bond is not acidic.
What is the use of aldehydes?
Formaldehyde is used as plant germicides, insecticides, and fungicides in the embalming, tanning, and processing of glues and polymeric items. It is also used for modelling and drug checking. Formaldehyde forms Bakelite when combined with phenol and is used in paints, coatings, and adhesives.
What are natural aldehydes?
Aldehydes are a class of reactive, organic compounds that can be found in natural products such as cinnamon bark (cinnamaldehyde) and vanilla bean (vanillin) as well as in laboratories.
For any further queries call our mentors at BYJU’S – The Learning App

Comments