Aim:
To prepare the organic compound Dibenzal acetone from benzaldehyde and acetone in the presence of sodium hydroxide.
Table of Contents
- Theory
- Materials Required
- Apparatus Setup
- Procedure
- Observations
- Results and Discussion
- Precautions
- Frequently Asked Questions – FAQs
Theory
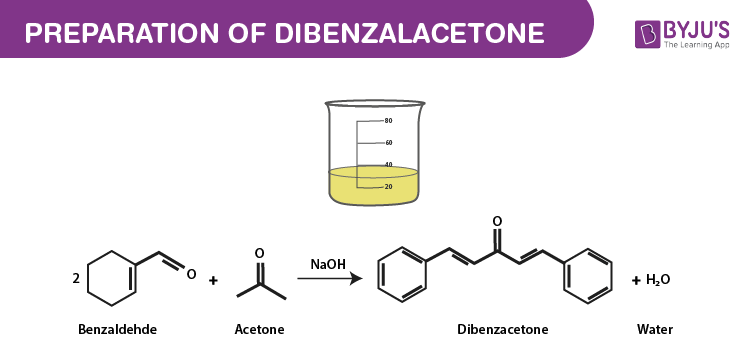
Aromatic aldehyde undergoes condensation reaction with aldehyde or ketone which contain alpha hydrogen atoms in the presence of an alkali. This reaction is called Claisen-Schmidt reaction. According to claisen aldehydes in the presence of sodium hydroxide can condense with another aldehyde or ketone eliminating a water molecule. Thus, moles of benzaldehyde condense with one mole of acetone to give Dibenzal acetone.
| Also Read: Preparation of Dibenzal Acetone Viva Questions |
The chemical reaction is given below.

In the same way, benzaldehyde condenses with acetaldehyde to give cinnamic aldehyde and one equivalent of acetone to give benzal-acetone.
Other names – Dibenzylideneacetone, 1,5-Diphenylpenta-1,4-dien-3-one, trans,trans-Dibenzylideneacetone
Materials Required
- Benzaldehyde
- Acetone
- Sodium hydroxide solution
- Methylated spirit
- Dilute hydrochloric acid
- Ether
- Beaker
- Funnel
- Conical flask
- Filter papers
Apparatus Setup
Procedure
- Take a conical flask add 10ml freshly distilled benzaldehyde and 20ml of acetone.
- Place the flask in cold water bath and then add 2.5ml sodium hydroxide drop wise with constant stirring.
- Maintain the temperature at 30oC.
- After the complete addition of sodium hydroxide stir the mixture for 2 hours.
- Add dilute hydrochloric acid to the reaction mixture and then transfer to a 250ml separating funnel.
- Add 20ml of chloroform/ether to the mixture and shake thoroughly.
- Shake the mixture thoroughly, remove the organic layer and repeat the process twice.
- Cool the mixture in ice-water. Dibenzal acetone separates initially as a fine emulsion and then forms yellow crystals.
- Distil the residual portion under pressure and collect the fraction boiling at 150oc.
- Wash the pale yellow crystals with cold water, dry and crystallize them with ethanol.
Observations
| Colour of the crystals | Pale yellow |
| Expected Yield | 4gm |
| Melting Point | 112oC |
Results and Discussion
The yield of Dibenzal Acetone is ______gm.
Precautions
- Whenever vigorous reaction is taking place inside the flask, release the pressure from time to time by opening the cork of the flask.
- Temperature should not be raised beyond 30oC.
- Ethanol and acetone should be kept away from the flame.
Keep visiting BYJU’S to learn more about class 12 CBSE chemistry practicals.
Frequently Asked Questions – FAQs
What is the IUPAC name for dibenzal acetone?
The IUPAC name for dibenzal acetone is 1,5-Diphenylpenta-1,4-dien-3-one.
Dibenzal acetone preparation is based on which naming reaction.
It is based on claisen condensation.
Why to lose the cork on the mouth of the flask during heating?
To prevent from building up the pressure in the flask while heating which may cause explosion.
What is the formula for dibenzal acetone?
The formula for dibenzal acetone is C17H14O.
What is condensation reaction?
A carbonyl condensation reaction takes place between two carbonyl partners and involves both nucleophilic addition and alpha substituted processes.

Comments