Everything that exists in the earth is a form of a matter which is further defined as any substance that occupies space and has mass. The matter is further divided into various forms such as solid, liquid and gas. Apart from these, it is also classified as pure substances and mixtures.
We will learn about the latter in this article.
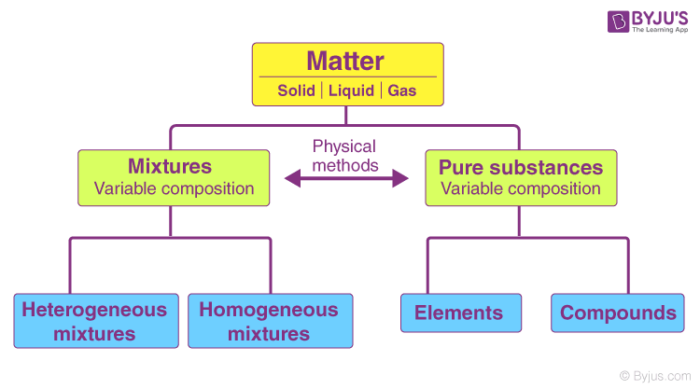
Classification of Matter – Pure Substances and Mixtures
Table of Contents
- What is Pure Substance?
- Characteristics and Properties Of Pure Substances
- Examples of Pure Substances
- Recommended Videos
- What is a Mixture?
- Characteristics And Properties Of Mixtures Or Impure Substance
- Example Of Mixtures
- Differences Between Pure Substances and Mixtures
- Frequently Asked Questions – FAQs
What is Pure Substance?
Pure substances are substances that are made up of only one kind of particle and have a fixed or constant structure.
Pure substances are further classified as elements and compounds.
An element is a substance that consists of only one type or kind of atom. An element is a pure substance as it cannot be broken down or transformed into a new substance even by using some physical or chemical means. Elements are mostly metals, non-metals or metalloids.
Compounds, on the other hand, are also pure substances when two or more elements are combined chemically in a fixed ratio. However, these substances can be broken down into separate elements by chemical methods.
Characteristics and Properties Of Pure Substances
- Pure substances are mostly homogeneous in nature containing only one type of atom or molecule.
- These substances mainly have a constant or uniform composition throughout.
- The substances have fixed boiling and melting points.
- A pure substance usually participates in a chemical reaction to form predictable products.
Examples of Pure Substances
All elements are mostly pure substances. A few of them include gold, copper, oxygen, chlorine, diamond, etc. Compounds such as water, salt or crystals, baking soda amongst others are also grouped as pure substances.
Recommended Videos

What is a Mixture?
A substance, on the other hand, is impure if it consists of different kinds of elements combined physically and not chemically. Impure substances are also called mixtures. Mixtures are further divided into homogenous or heterogeneous mixture.
- A homogeneous mixture occasionally called a solution, is comparatively unvarying in configuration or constant. Every unit of the mixture is like every other unit. For instance, if you liquefy sugar in water and blend it really well, your concoction is essentially the same, no matter where you sample it. This mixture contains two or more chemical substances.
- A heterogeneous mixture is a concoction whose configuration varies from spot to spot within the sample. For example, if you put a little amount of sugar in a vessel, add some sand, and then shake the jar a couple of times, your concoction doesn’t have the same configuration all throughout the jar. As the sand is heftier, there’s possibly more amount of sand at the bottom of the jar and more sugar at the top part. These mixtures can be identified visually and separated easily by physical means.
Characteristics And Properties Of Mixtures Or Impure Substance
- It does not have any specific properties, the properties of the mixture are a result of the average properties of all the constituents.
- It is formed as a result of a physical change.
- They have a variable composition.
- Their melting and boiling points differ.
Example Of Mixtures
Some common examples of mixtures include;
- Gas and gas like nitrogen and oxygen in the atmosphere.
- A solution like water and oil.
- Gas and liquid such as water.
- Solid and liquid such as sand and water
Differences Between Pure Substances and Mixtures
The differences between pure substances and mixtures are given below.
| Pure Substances | Mixtures |
| It cannot be broken down or separated into new products. | It can be separated using different separation methods. |
| Constant physical and chemical properties. | Mixtures have varying physical and chemical properties. |
| Pure substances are made up of a single element. | A mixture is a combination of two substances or elements. |
Frequently Asked Questions – FAQs
What is pure substance explain with example?
Every homogeneous mixture is a pure material. These substances mainly have a constant or similar composition throughout, no matter how small the sample size. Iron, steel, and water are examples of pure substances.
Is wood a pure substance?
It is a mixture since it consists of compounds such as cellulose, hemicellulose, and lignin that are composed of elements such as carbon, hydrogen, and oxygen. Wood is not a pure material.
What is meant by pure substance and mixture?
A pure substance is a single kind of matter that cannot be separated into other kinds of matter by any physical means. A pure substance always has a definite and constant composition. A mixture is a physical combination of two or more pure substances in which each substance retains its own chemical identity.
A pure substance is a single substance on its own. Elements and compounds are pure substances, but mixtures are not. Compounds are very different from the elements they contain. But a mixture resembles the substances it contains. The properties of the mixture are a kind of average of the properties of the substances in it. So a mixture of sugar and water is both sweet and wet.
What are the properties of substances?
Any characteristic that can be measured, such as an object’s density, color, mass, volume, length, malleability, melting point, hardness, odour, temperature, and more, are considered properties of matter.
What are the characteristics of pure solution?
Pure materials have a particular set of characteristics, such as boiling point, melting point, density, etc. All of them are homogeneous, i.e., their distribution in the bulk is uniform. Pure substances are both elements and compounds.

THAT WAS VERY HELPFUL TO ME.
EXCELLENT ANSWERS
These Notes were very helpful,
Thank you so much Byjus
This videos are always very helpful and intresting
It’s was very helpful
YES THESE NOTES ARE AWESOME AND VERY HELPFUL,
THANK YOU SO MUCH BYJUS
It was very helpful . Thank you for sharing. God Bless!
Thank you for keeping it simple, and resourceful! 🙂
Thank you so much this helps alot!
Thank you byju
this notes was very helpful to me thank you byjus
thank u byjus
This notes are very helpful to me
Thank u so much byjus
This helped me a lot, thank you!
Very helpful, thank you so much!
Yes these notes are very helpful
Thank you so much! byjus