The name Reformatsky reaction is mostly kept in honour of Russian chemist Sergey Nikolaevich Reformatsky who discovered the reaction in 1887. This is a type of reaction that occurs between an alpha‐haloester and a carbonyl compound which can be a ketone, aldehyde, or an ester. The reaction mostly takes place in the presence of zinc. The reaction is also a representation of the extended reactions between carbonyl compounds with a dialkylzinc or an alkyl zinc halide.
One advantage of this reaction is that the isolation of the organozinc compound is not required. During the reaction process, a new carbon‐carbon linkage is created along with the formation of an organozinc halide and decomposition due to the presence of dilute acids.
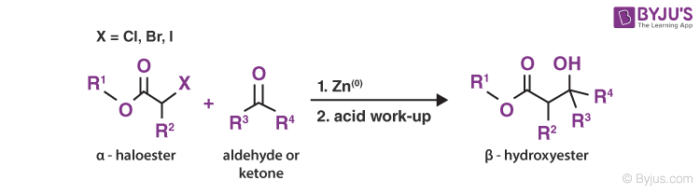
The yields in the Reformatsky reaction are generally improved if the reaction is carried out in two steps.
- First, the alpha bromo ester is converted into an organozinc bromide
- By reaction with zinc in pure and dry dimethoxyethane.
This derivative is apparently formed in almost quantitative yield.
Table of Contents
Definition
According to the general definition, the Reformatsky reaction is defined as an organic reaction that is used to convert ketone or an aldehyde and α-half ester to a β-hydroxy ester by using metallic zinc and acid workup. Here, inert solvent like diethyl ether or THF (tetrahydrofuran) is often used as a solvent for the reaction.
Condensation reaction of carbonyl compounds with alpha haloester in presence of zinc metal is known as the Reformatsky reaction.
The solvent most often used in this reaction is benzene or ether or a benzene ether mixture.
Mechanism of the Reaction
Let us look at how the reaction starts and what happens during the reaction.
- The Reformatsky reaction usually commences with the oxidative addition or insertion of the zinc into the carbon-halogen bond of α-haloester.
- The main purpose of using zinc is that it allows the generation of an enolate even without using a Bronsted base which normally condenses with the ketone or aldehyde itself.
- After the insertion, the compounds coordinate with each other leading to the formation of a dimer. This compound further experiences a rearrangement which results in the emergence of two zinc enolates.
- Then, the oxygen of the ketone or aldehyde coordinates to the zinc and a new rearrangement is formed where the two reagents now have a carbon-carbon bond between them.
- Following that, an acid workup splits the zinc and oxygen bond to generate zinc(II) salt and β-hydroxy ester as the final products.
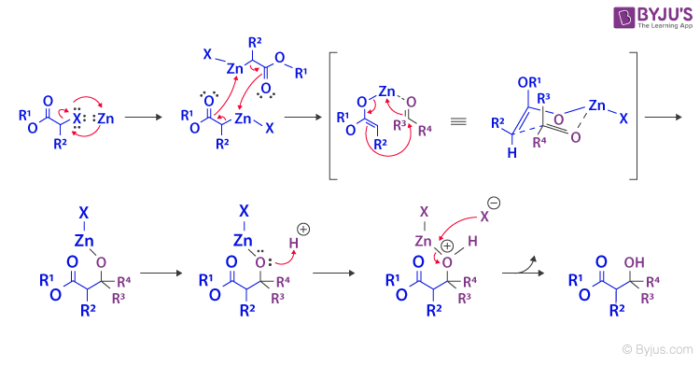
Notably, the product α-hydroxy esters are important substances required for natural product synthesis and in pharmaceuticals.
Advantages
Some significant advantages of this reaction are;
- Reformatsky reaction can be conducted with highly hindered ketones. The reaction facilitates the successful addition of nucleophiles to the delta positive carbon atom of a ketone.
- Reformatsky mechanism can be easily adapted for intramolecular aldol reactions.
- The organozinc halide reagents used in the Reformatsky Reaction are relatively stable. They are also available commercially.
- Reformatsky results in the isolation of beta-hydroxy ester.
- Another merit of the Reformatsky reaction is convenience since the reaction is an alternative to the reaction of an aldehyde or a ketone with the preferred lithium enolate of an ester.
- Yields of Reformatsky were found to be improved by using freshly prepared zinc powder a heated column of zinc dust, copper-zinc couple, acid-washed zinc and trimethylchlorosilane.
Frequently Asked Questions – FAQs
What is the name reaction in organic chemistry?
A name reaction is a chemical reaction named after its creators or discoverers. Hundreds of such reactions are well-known enough to be named after individuals, within the tens of thousands of organic reactions that are known.
Is combustion an organic reaction?
Easy, exothermic chemical reaction of a fuel (usually oxygen) with an oxidizing agent. Carbon dioxide and water are created by complete combustion of a hydrocarbon like methane. Carbon monoxide is produced by incomplete combustion.
Is polymerization in organic reaction?
Polymerization is a mechanism by which an organic compound reacts with itself to create a compound of high molecular weight consisting of the initial compound’s repeating units. The reaction of ethene with sulphuric acid is an example of a cation-initiated polymerization.
How does a catalyst speed up a reaction?
An alternative reaction mechanism that has a lower activation energy than the uncatalysed reaction is given by a catalyst. However, since more particles have energy greater than the activation energy, the number of successful collisions increases, so there are more successful collisions.
Which reaction is exothermic?
Exothermic reactions are reactions or processes which, typically in the form of heat or light, release energy. Energy is produced in an exothermic reaction when the total energy of the materials is below the total energy of the reactants.


Comments