What is Resorcinol?
Resorcinol is an organic compound with the chemical formula C6H6O2. It is one of the three isomeric benzenediols which is white and soluble in water. It is also known as Resorcin or m-Dihydroxybenzene. It is a 1,3-isomer of benzenediol. It is a benzenediol that is benzene dihydroxylated at positions number 3 and 1. It functions as a sensitizer and an erythropoietin inhibitor.
Resorcin is a crystalline solid very white and soluble in water, ether, and alcohol. It has a faint odour with a sweetish to bitter taste. It does not dissolve carbon disulfide and chloroform. It turns pink when exposed to light if it is not in its pure form. It burns, however, difficult to ignite. It is widely used in the making pharmaceuticals and plastics.
Table of Contents
- Resorcinol Properties
- Structure of Resorcinol (C6H6O2)
- Uses of Resorcinol (C6H6O2)
- Production of Resorcinol
- Frequently Asked Questions
Resorcinol Properties
| Resorcinol | C6H6O2 |
| Molecular Weight of Resorcinol | 110.1 g/mol |
| Density of Resorcinol | 1.28 g/cm3 |
| Boiling Point of Resorcinol | 277 °C |
| Melting Point of Resorcinol | 110 °C |
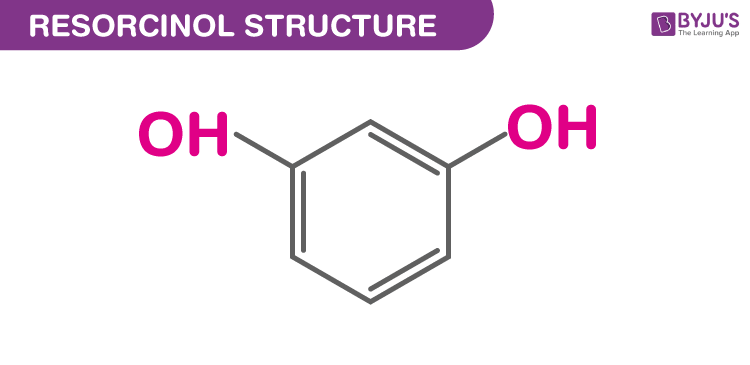
Structure of Resorcinol (C6H6O2)
Uses of Resorcinol (C6H6O2)
- Resorcinol is used as a disinfectant or an antiseptic in pharmaceutical products.
- It is used to treat skin infections such as seborrheic dermatitis, psoriasis, calluses, eczema, warts, and acne.
- It is used in the manufacturing of resins.
- It is an analytical reagent used to determine the quality of ketoses.
Production of Resorcinol
m-Dihydroxybenzene can be synthesised in various ways. Some of them are discussed here. Dialkylation of benzene with propylene to obtain 1,3-diisopropyl benzene. Hock rearrangement and oxidation of this obtained product give resorcinol and acetone.
It can be produced by melting resins such as asafoetida, and galbanum with KOH (potassium hydroxide), or by the distillation process of Brazilwood extract.
It may be also prepared by melting benzene-1,3-disulfonic acid, phenol-3-sulfonic acid, and 3-iodophenol, in the presence of potassium carbonate (K2CO3) by the action of nitrous acid (HNO2) on 1,3-diaminobenzene or on 3-aminophenol. Many para and ortho compounds of the aromatic series such as benzene-para-disulfonic acid, and bromophenols also yield resorcinol on fusion with KOH (potassium hydroxide).
Frequently Asked Questions
What is resorcinol used for?
Resorcinol has the ability to break down rough, hardened, or scaly skin. In its topical form, it is also used to treat eczema, acne, psoriasis, corns, seborrhea, calluses, warts, and several other disorders of the skin.
Comment on the antibacterial and antifungal properties of resorcinol
The antibacterial and antifungal activities may result from protein precipitation. However, the keratolytic activity may contribute to the antifungal effect, as removal of the stratum corneum suppresses fungal growth. Absorption: Resorcinol can be absorbed from ulcerated surfaces or via the skin.
What is resorcinol derived from?
Resorcinol, also known as m-dihydroxybenzene, is a phenolic compound which is used in the manufacture of resins, colourants, plastics, medicinal goods and many other organic chemical compounds. It is formed in large amounts by sulfonising benzene with fuming sulfuric acid and by fusing caustic soda to the resulting benzenedisulfonic acid.
Learn more about the different applications, structure, and properties of Resorcinol from the expert faculties at BYJU’S – India’s largest education company.

Comments