Table of Contents
What is Distillation?Role of Raoults Law and Daltons LawTypes of DistillationImportant Applications
What is Distillation?
Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a particular component in the mixture or to obtain (almost) pure components from the mixture. The process of distillation exploits the difference in the boiling points of the components in the liquid mixture by forcing one of them into a gaseous state.
It is important to note that distillation is not a chemical reaction but it can be considered as a physical separation process. An illustration describing the laboratory setup that is generally used to execute this process is provided below.

The distillation performed on a laboratory scale often uses batches of the liquid mixture whereas industrial distillation processes are generally continuous, requiring a constant composition of the mixture to be maintained.
Role of Raoult’s Law and Dalton’s Law
The temperature at which the vapor pressure of a liquid becomes equal to the pressure of the surrounding area is known as the boiling point of that liquid. At this temperature point, the liquid is converted into its vapor form via the formation of vapor bubbles at its bulk.
It is important to note that the boiling point of the liquid changes with the surrounding pressure. For example, the boiling point of water at sea level is 100oC but its boiling point at an altitude of 1905 meters is 93.4oC (since the atmospheric pressure is relatively lower at high altitudes).
For a mixture of liquids, the distillation process is dependent on Dalton’s law and Raoult’s law. As per Raoult’s law, the partial pressure of a single liquid component in an ideal liquid mixture equals the product of the vapor pressure of the pure component and its mole fraction. According to Dalton’s law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all the constituent gases.
When a mixture of liquids is heated, the vapor pressure of the individual components increases, which in turn increases the total vapor pressure. Therefore, the mixture cannot have multiple boiling points at a given composition and pressure.
Why is it Impossible to Completely Purify a Mixture by Distillation?
At the boiling point of a mixture of liquids, all the volatile constituents boil. However, the quantity of a constituent in the resulting vapor is based on its contribution to the total vapor pressure of the mixture. This is why the compounds with higher partial pressures can be concentrated in the vapors whereas the compounds having low partial pressures can be concentrated in the liquid.
Since a component in the mixture cannot have zero partial pressure, it is impossible to obtain a completely pure sample of a component from a mixture via distillation. However, samples of high purity can be obtained when one of the components in the mixture has a partial pressure which is close to zero.
Types of Distillation
Some important types of distillation include:
- Simple distillation
- Fractional distillation
- Steam distillation
- Vacuum distillation
- Air-sensitive vacuum distillation
- Short path distillation
- Zone distillation
Simple Distillation
- Simple distillation involves heating the liquid mixture to the boiling point and immediately condensing the resulting vapors.
- This method is only effective for mixtures wherein the boiling points of the liquids are considerably different (a minimum difference of 25oC).
- The purity of the distillate (the purified liquid) is governed by Raoult’s law.
Fractional Distillation
Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It involves several vaporization-condensation steps (which takes place in a fractioning column). This process is also known as rectification. The apparatus required to perform a fractional distillation on a mixture is listed below.
- Round-bottom flask or distilling flask
- A source of heat, which can be a fire or a hot bath.
- Receiving flask to collect the condensed vapors
- Fractioning column
- Thermometer to measure the temperature in the distilling flask
- Condenser
- Standard Glassware.
When heated, the liquid mixture is converted into vapors that rise into the fractioning column. The vapors now cool and condense on the walls of the condenser. The hot vapors emanating from the distilling flask now heat the condensed vapor, creating new vapors.
Many such vaporization-condensation cycles take place and the purity of the distillate improves with every cycle. An illustration depicting a fractional distillation setup is provided below.
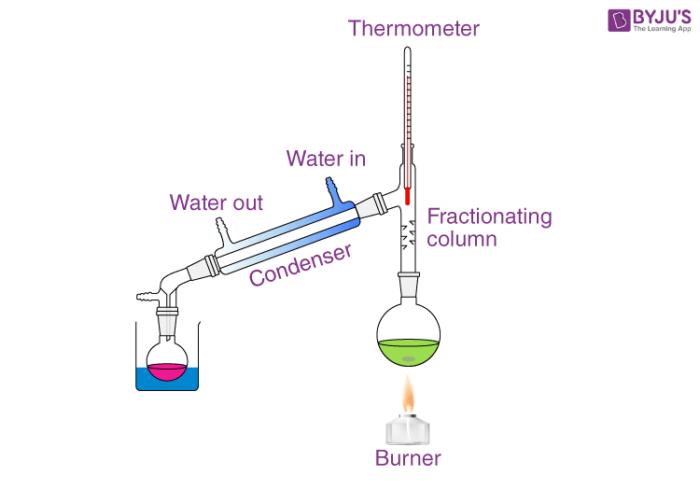
Commonly used condensers in laboratories include Liebig condensers and Graham condensers.
Steam Distillation
- Steam distillation is often used to separate heat-sensitive components in a mixture.
- This is done by passing steam through the mixture (which is slightly heated) to vaporize some of it. The process establishes a high heat-transfer rate without the need for high temperatures.
- The resulting vapor is condensed to afford the required distillate.
- The process of steam distillation is used to obtain essential oils and herbal distillates from several aromatic flowers/herbs.
Vacuum Distillation
- Vacuum distillation is ideal for separating mixtures of liquids with very high boiling points.
- In order to boil these compounds, heating to high temperatures is an inefficient method. Therefore, the pressure of the surroundings is lowered instead.
- The lowering of the pressure enables the component to boil at lower temperatures. Once the vapor pressure of the component is equal to the surrounding pressure, it is converted into a vapor.
- These vapors are then condensed and collected as the distillate. The vacuum distillation method is also used to obtain high-purity samples of compounds that decompose at high temperatures.
Air-Sensitive Vacuum Distillation
For compounds that are sensitive to air and readily react with it, the vacuum distillation process is carried out but the vacuum must be replaced with an inert gas once the process is complete. Such a process is often referred to as air-sensitive vacuum distillation.
Short Path Distillation
Short path distillation is used to purify a small quantity of a compound that is unstable at high temperatures. This is done under lowered pressure levels and generally involves the distillate traveling a very small distance before being collected (hence the name ‘short path’). The reduced distance traveled by the distillate in this method also reduces the wastage along the walls of the apparatus.
Zone Distillation
The process of zone distillation involves the partial melting of a substance and the condensation of the resulting vapors to obtain a pure distillate. This is carried out in a long container with the help of a zone heater.
Important Applications
The method of distillation has a considerable history, dating back to 3000 BC. Evidence suggests that the distillation of alcohol was developed as far back as the 9th century. Some important applications of distillation are listed below.
- Distillation plays an important role in many water purification techniques. Many desalination plants incorporate this method in order to obtain drinking water from seawater.
- Distilled water has numerous applications, such as in lead-acid batteries and low-volume humidifiers.
- Many fermented products such as alcoholic beverages are purified with the help of this method.
- Many perfumes and food flavorings are obtained from herbs and plants via distillation.
- Oil stabilization is an important type of distillation that reduces the vapor pressure of the crude oil, enabling safe storage and transportation.
- Air can be separated into nitrogen, oxygen, and argon by employing the process of cryogenic distillation.
- Distillation is also employed on an industrial scale to purify the liquid products obtained from chemical synthesis.
To learn more about distillation and other related topics, such as sublimation, register with BYJU’S and download the mobile application on your smartphone.
Recommended Videos
Types of Distillation

Frequently Asked Questions on Distillation
What is distillation?
Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a particular component in the mixture or to obtain (almost) pure components from the mixture.
What is the boiling point?
The temperature at which the vapour pressure of a liquid becomes equal to the pressure of the surrounding area is known as the boiling point of that liquid.
Does the boiling point of a liquid vary with pressure?
Yes, the boiling point of a liquid varies with pressure. For example, the boiling point of water at sea level is 100℃ but its boiling point at an altitude of 1905 meters is 93.4℃ (As the atmospheric pressure is relatively lower at high altitudes).
Where do we use fractional distillation?
Fractional distillation is primarily used in separating liquid with comparable boiling points. It involves several vaporization-condensation steps taking place in a fractioning column.
What is zone distribution?
Zone distillation involves the partial melting of a substance and the condensation of the resulting vapours to obtain a pure distillate. This is carried out in a long container with the help of a zone heater.

Good
Thanks for the brilliant information especially chemistry notes.