Point defects explain about the imperfections of solids along with the types of point defects. Crystalline solids are formed by joining many small crystals. Different types of defects are found in crystals after the process of crystallization.
Point defects are accounted for when the crystallization process occurs at a very fast rate. These defects mainly happen due to deviation in the arrangement of constituting particles. In a crystalline solid, when the ideal arrangement of solids is distorted around a point/ atom it is called a point defect.
Defects or Imperfections in crystalline solid can be divided into four groups namely line defects, point defects, volume defects and surface defects. Historically, crystal point defects were first regarded in ionic crystals, not in metal crystals that were much simpler.
There are 3 types of point defects:
- Stoichiometric defect
- Frenkel defect
- Schottky defect
Table of Contents
- Stoichiometric Defect
- Frenkel Defect
- Schottky Defect
- Types of Non-Stoichiometric Defect
- Frequently Asked Questions – FAQs
1. Stoichiometric Defect:
In this kind of point defect, the ratio of positive and negative ions (Stoichiometric) and electrical neutrality of a solid is not disturbed. Sometimes it is also known as intrinsic or thermodynamic defects.
Fundamentally, they are of two types:
- Vacancy defect: When an atom is not present at their lattice sites, then that lattice site is vacant and it creates a vacancy defect. Due to this, the density of a substance decreases.
- Interstitial defect: It is a defect in which an atom or molecule occupies the intermolecular spaces in crystals. In this defect, the density of the substance increases.
A non-ionic compound mainly shows vacancy and interstitial defects. An ionic compound shows the same in Frenkel and Schottky defect.
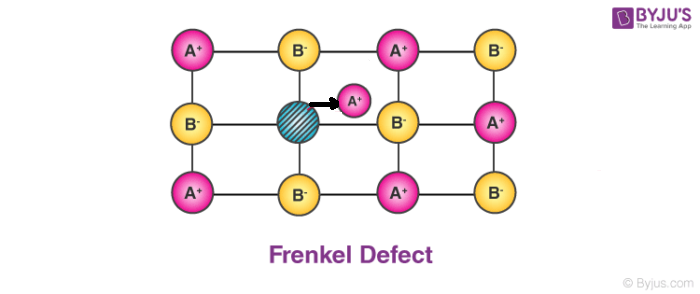
2. Frenkel Defect:
In ionic solids generally, the smaller ion (cation) moves out of its place and occupies an intermolecular space. In this case, a vacancy defect is created on its original position and the interstitial defect is experienced at its new position.
- It is also known as dislocation defect.
- The density of a substance remains unchanged.
- It happens when there is a huge difference in the size of anions and cations.
- Example: ZnS and AgCl.
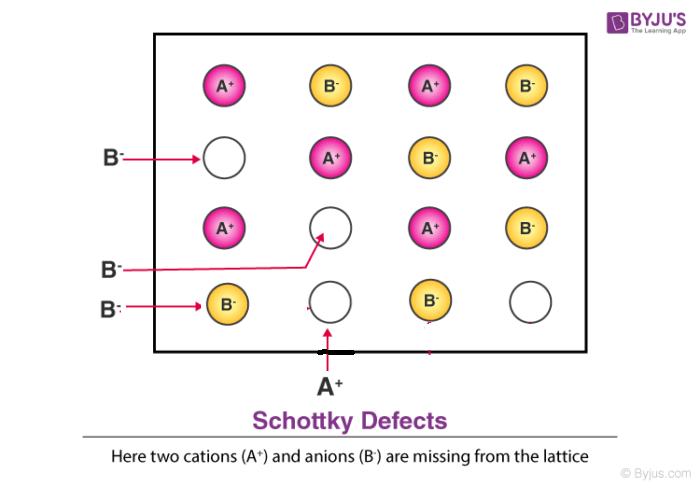
3. Schottky Defect
- This kind of vacancy defects is found in Ionic Solids. But in ionic compounds, we need to balance the electrical neutrality of the compound so an equal number of anions and cations will be missing from the compound.
- It reduces the density of the substance.
- In this, the size of cations and anions are of almost the same.
- Impurity Defect: Let’s understand the impurity defect by an example. If molten NaCl is crystallized with SrCl2 compound then the Sr2+ ions replace two Na+ ions and occupy the place of one Na+ In this way the lattice site of one Na+ is vacant and it creates an impurity defect.
- Non-Stoichiometric Defect: In this defect, the cations and anions ratio is disturbed either because of adding or removing of ions.
Types of Non-Stoichiometric Defect:
- Metal deficiency defect: In this, the solids have less number of metals relative to the described Stoichiometric proportion.
- Metal excess defect: There are two types of metal excess defect:
- Metal excess defect due to anionic vacancies: This occurs due to the absence of anions from its original lattice site in crystals. Therefore, instead of anions, electrons occupy their position
- Metal excess defect due to the presence of extra cations at interstitial sites: Here, on heating the compound, it releases extra cations. These cations occupy the interstitial sites in crystals and the same number of electrons goes to neighbouring interstitial sites.
There is so much to learn about the crystalline solids, its structure and its defects. This is just the overview of point defects in solids.
Recommended Videos

Frequently Asked Questions – FAQs
What are point defects with examples?
Point defects are zero-dimensional lattice defects, meaning they have no lattice structure in any dimension. Impurity atoms in a pure metal, vacancies, and self-interstitials are examples of point defects.
What are point defects in solids?
Defects that occur only at or near a single lattice point are known as point defects. They are not physically extended in any way. In most cases, there are no clear constraints on how small a point defect can be. These flaws, on the other hand, usually only entail a few extra or missing atoms.
Which is a line defect?
Line defects, also known as dislocations, are lines in a solid along which entire rows of atoms are arranged abnormally. The ensuing spacing irregularity is most apparent along a line known as the line of dislocation. Solids can be weakened or strengthened by line faults.
What is point defect in crystal?
The simplest defects that can be found in any crystal phase are point defects. They are focused on single crystal structure sites, which may be consistently inhabited by chemical species or otherwise unoccupied sites of the vacant interstitial sublattice.
Is dislocation a point defect?
The interaction between a dislocation and a point defect is modelled as follows: a dislocation, which is considered to be straight, is located at a glide plane projected distance from a point defect, which is located at a height z above the glide plane.
To know more about solids download BYJU’S – the learning app.


Comments