Rosenmund Reduction Mechanism explains the way acyl chlorides are selectively reduced into aldehydes. Karl Wilhelm Rosenmund first reported this reaction in 1918, thus the reaction was named after him. The Rosenmund reaction is a hydrogenation process where molecular hydrogen reacts with the acyl chloride in the presence of catalyst – palladium on barium sulfate. Barium sulfate reduces the activity of the palladium due to its low surface area, thereby preventing over reduction. To prevent further reduction, (as in the case of more reactive acyl chlorides), poison can be added to totally deactivate the palladium catalyst.
Common poisons used to limit palladium activity in the Rosenmund technique are thioquinanthrene and thiourea. The need for deactivation arises because the subsequent aldehyde formed from the reduction of the acyl chloride will also be reduced to primary alcohol by the system. This primary alcohol would then react with the remaining acyl chloride, forming an ester. The Rosenmund catalyst (palladium on barium sulfate) is prepared by the reduction of palladium(II) chloride solution with reducing agent formaldehyde in the presence of barium sulfate.
Rosenmund Reduction
As discussed in the introduction, the Rosenmund reduction is a reaction where acid chlorides are converted into aldehydes by employing hydrogen gas over palladium poisoned by barium sulfate. An example of this catalytic hydrogenation of acyl chlorides forming aldehydes is shown below.
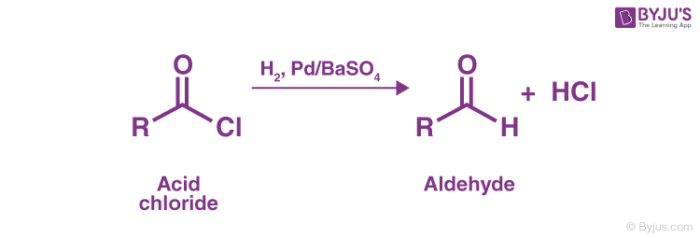
Due to the high reactivity of hydrogen gas, it readily initiates a substitution in the acyl chloride, forming HCl and the required aldehyde.
Mechanism of Rosenmund Reduction
Step 1
Hydrogen gas (in the presence of the Rosenmund Catalyst) is passed through acyl chloride, resulting in the formation of an aldehyde and hydrochloric acid.
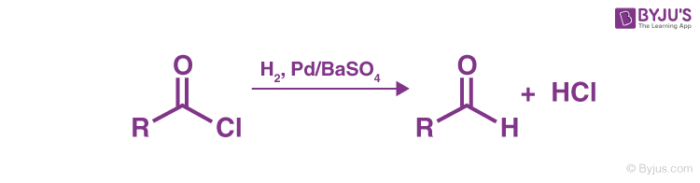
Step 2
The resulting aldehyde formed undergoes another reaction with the palladium over barium sulfate. This leads to the formation of alcohol which further generates an alkane.
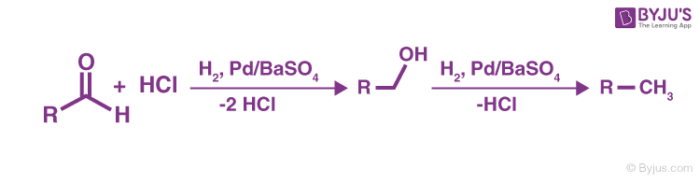
Step 3
The Rosenmund catalyst is poisoned to stop further reduction once the desired product is obtained. A variety of poisons can be employed for this process. An example of the method to totally deactivate the palladium over barium sulfate catalyst is shown below:
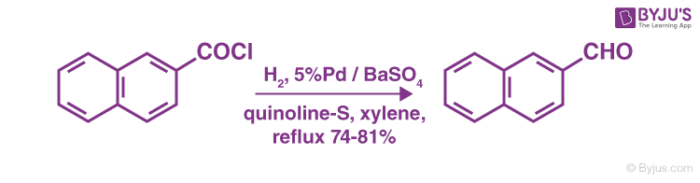
To conclude, the 3 step Rosenmund reduction mechanism can be employed for the catalytic hydrogenation of acyl chlorides to enable the formation of aldehydes. A few of the side products of this process can be avoided if the reaction is conducted in anhydrous solvents.

Comments