Rutherford atomic model was the first step in the evolution of the modern atomic model. Ernest Rutherford was a keen scientist who worked to understand the distribution of electrons in an atom. He performed an experiment using alpha particles and gold foil and made the following observations:
- Most of the alpha particles passed straight through the gold foil.
- There was a deflection of the alpha particles by a small angle.
- Very small amount of alpha particles rebounded.
Table of Content
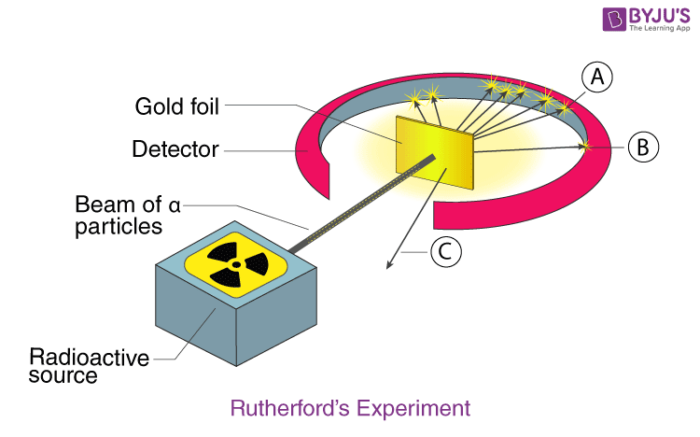
From his experiment, he came to the following conclusions:
- Most of the space in an atom is empty.
- The space occupied by the positive charges is very small.
- The positive charges and mass of the atom were concentrated in a very small volume within an atom.
From these conclusions, he calculated that the radius of the nucleus is around 105 times less than that of the atom.
Rutherford Atomic Model
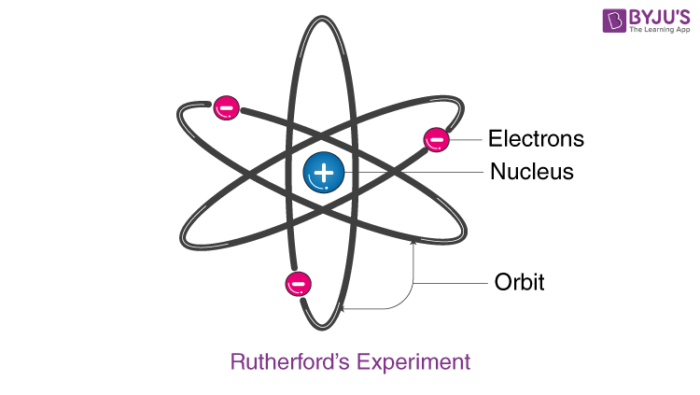
Rutherford developed a nuclear model of the atom on the basis of his experiment and observations. The Rutherford atomic model has the following features:
- The centre of an atom is called the nucleus. It is positively charged and almost all mass of the atom resides in it.
- Electrons spin around the nucleus in a circular path.
- Comparatively, the size of the nucleus is smaller than the size of the atom.
Drawbacks of Rutherford Atomic Model
As before, the Rutherford atomic model was also challenged and questioned by many. Rutherford atomic model failed to explain about the stability of electrons in a circular path.
As per Rutherford’s model, electrons revolve around the nucleus in a circular path. But particles that are in motion on a circular path would undergo acceleration, and acceleration causes radiation of energy by charged particles. Eventually, electrons should lose energy and fall into the nucleus. And this points to the instability of the atom. But this is not possible because atoms are stable. Hence, Rutherford failed to give an explanation on account of this.
To learn more about the Rutherford atomic model with video lessons, download BYJU’S – The Learning App.
Recommended Videos

Frequently Asked Questions On Drawbacks of Rutherford Atomic Model
What is the rutherford atomic model?
Rutherford atomic model is a nuclear model of the atom based on his experiment and observations. He worked to understand the distribution of electrons in an atom. He performed an experiment using alpha particles and gold foil.
What are the drawbacks of the rutherford atomic model?
Rutherford’s atomic model failed to explain the stability of electrons in a circular path. He stated that electrons revolve around the nucleus in a circular path, but particles in motion would undergo acceleration and cause energy radiation. Eventually, electrons should lose energy and fall into the nucleus. But it never happens.
What were the observations of the rutherford atomic model?
Rutherford observed that most of the alpha particles passed straight through the gold foil, while some of them were deflected by a small angle, and a very few rebounded.
What was the conclusion of the rutherford atomic model?
Rutherford concluded that most of the space in an atom is empty, a positively charged dense particle is present inside the atom that occupies a little space and most of the mass of an atom is concentrated over there.
What are the features of the rutherford atomic model?
Rutherford’s model states that the centre of an atom is the nucleus which is positively charged, occupying most of the atom’s mass. He also explained that electrons spin around the nucleus in a circular path.

Thanks for giving information
Thank you so much. Tomorrow is chemistry half yearly exam. This helped me a lot.
Good
Nice
He used full for me
Very ravishing and wonderful explained.