What is Sodium Hypochlorite?
Sodium hypochlorite is a chemical compound with the chemical formula NaClO.
It is also known as liquid bleach. It consists of hypochlorite anion and sodium cation. It usually appears as a pale greenish yellow dilute solution. It is an anhydrous unstable compound which can decompose explosively. It has a sweetish and chlorine-like odour. It is widely used as a cleaning agent or disinfectant and bleaching agent. It is a widely used household chemical.
Properties of Sodium Hypochlorite – NaClO
| NaClO | Sodium hypochlorite |
| Molecular Weight/ Molar Mass | 74.44 g/mol |
| Density | 1.11 g/cm³ |
| Boiling Point | 101 °C |
| Melting Point | 18 °C |
Sodium Hypochlorite Structure – Bleach
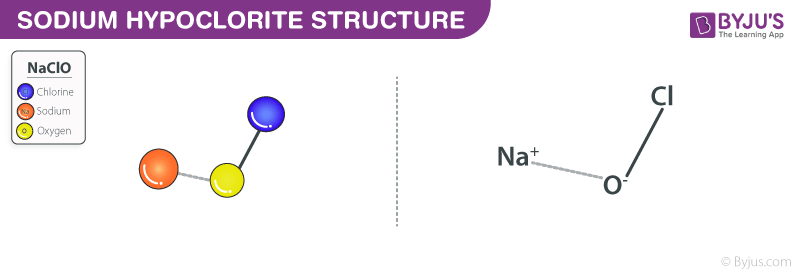
Sodium Hypochlorite Structure – Bleach
NaClO Uses (Sodium hypochlorite)
- It is used as a key ingredient in laundry bleach
- It is used as a bleaching agent
- It is used in textile industries
- It is used as an oxidizing agent
- It is used in detergent industries
- It is used in the refining of petroleum products
- It is used in paper industries
- It is used in wastewater treatment
- It is used as a disinfectant
- It is used in food processing to sanitize the food preparation equipment
- It is used in swimming pools to keep the infectious agents at bay
Frequently Asked Questions
Is sodium hypochlorite the same as bleach?
Sodium hypochlorite is a solid white powder, but dissolved in water is more widely used. Sodium hypochlorite solutions are generally called bleach, though household bleach also contains small quantities of many other compounds including sodium hydroxide and calcium hypochlorite.
What is the common name for sodium hypochlorite?
Chlorine.-Bleach. Sodium hypochlorite is a strong liquid oxidizing agent and has a greenish or yellowish hue. It is usually called bleach, because it is the active ingredient in bleach. The chemical formula is NaClO and consists of one atom of sodium (Na), one atom of chlorine (Cl) and one atom of oxygen (O).
Will sodium hypochlorite kill weeds?
Sodium hypochlorite, also called bleach, has an average pH of 11. Anything with a pH of 11 can kill grass and other plants, the majority of which need a mid-range soil pH: around 7.0.
Is sodium hypochlorite an ionic compound?
Formula and structure: Sodium hypochlorite’s chemical formula is NaClO, and its molar mass is 74.44 g / mol. This is an ionic compound consisting of the hypochlorite anion (ClO-) bonded sodium metal cation (Na+)
Is sodium hypochlorite harmful to humans?
Once sodium hypochlorite has been swallowed the symptoms are stomach ache, a burning feeling, coughing, nausea, sore throat and vomiting. Sodium hypochlorite causes redness and discomfort on skin or hair. The skin can become responsive, after prolonged exposure. Sodium hypochlorite is toxic for animals that use water.
Also, Read:
| Potassium Permanganate | Potassium Chloride |
| Calcium Hydroxide | Ammonium Sulfate |
Learn more about the properties, production and the structure of NaClO from the expert faculties at BYJU’S.

Comments