What is Sodium Sulphate?
Sodium sulphate (Na2SO4) is the sodium salt of sulfuric acid.
Anhydrous sulphate is a white crystalline solid also known as the mineral thenardite, while the decahydrate Na2SO4.10H2O has been known as Glauber’s salt or mirabilis.
Na2SO4.7H2O is transformed to mirabilite when it is cooled. Mirabilite is the natural mineral form of the decahydrate. About two-thirds of the world’s production of sodium sulphate is obtained from mirabilite. It is also produced from by-products of chemical processes such as hydrochloric acid production.
Meaning of Anhydrous
Anhydrous literally means “no water.” In chemistry, substances without water are labelled as anhydrous. The term is most often applied to crystalline substances after the water of crystallisation is removed.
The name and function of salt compounds change with the presence or absence of water in their crystalline structures. For example, sodium sulphate, NaSO4, is called anhydrous sodium sulphate when free of water and is used as a drying material. That same compound in its decahydrate iteration is called “Glauber’s salt,” and is used to make glass. A molecule with no water molecule in it usually absorbs moisture from the salt to which it comes in contact with and hence use as a dehydrating agent.
Table of Content
- Structure of Sodium Sulphate
- Properties of Sodium Sulphate
- Preparations of Sodium Sulphate
- Resources of Sodium Sulphate
- Uses of Sodium Sulphate
- FAQs
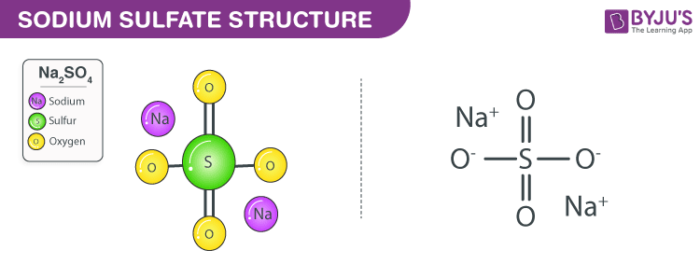
Structure of Sodium Sulphate

Properties of Sodium Sulphate
| Molecular formula | Na2SO4 |
| Molecular weight | 142.04 gm/mole (anhydrous), 322.20 gm /mole (decahydrate) |
| Appearance | White crystalline solid |
| Odour | Odourless |
| Boiling point | 1429 oC(anhydrous) |
| Flashpoint | 800 oC |
| Melting point | 884 oC (anhydrous), 32.4 0C (decahydrate) |
| Density | 2.664 gm/ml (anhydrous), 1.464 gm/ml (decahydrate) |
| Refractive index | 1.468 (anhydrous), 1.394 (decahydrate) |
| Solubility | Soluble in water, glycerol and hydrogen iodide and insoluble in ethanol |
Preparations of Sodium Sulphate
In 1625, Johann Rudolf Glauber discovered sodium sulphate from Austrian spring water, so its hydrate form is known as Glauber’s salt. Due to its medicinal properties, he named it sal mirabilis (miraculous salt).
One-third of the world’s sodium sulphate is produced as a by-product of other processes in the chemical industry. It is produced by the reaction of sodium chloride and sulphuric acid.
2 NaCl + H2SO4 → 2 HCl + Na2SO4
The crystals were used as a general-purpose laxative, until the 1900s. By reaction with potassium carbonate or potash, Glauber’s salt was used as a raw material for the industrial production of soda ash in the 18th century. In the nineteenth century, the demand for soda ash increased, so the large-scale Leblanc process which produced synthetic sodium sulphate became the principal method of soda ash production.
At dietary levels, excretion is mainly in the urine. Sulphates are found in all body cells, with the highest concentrations in connective tissues, bone, and cartilage. Sulphates play a role in several important metabolic pathways, including those involved in detoxification processes.
There are two types of sodium sulphate natural and by-product, also known as synthetic.
- Natural sodium sulphate is produced from naturally occurring brines and crystalline deposits found in California and Texas.
- It is also found as a constituent of saline lakes, such as the Great Salt Lake in Utah. Synthetic sodium sulphate is recovered as a by-product of various manufacturing processes.
- Both types of sodium sulphate have several important and useful applications in various consumer products.
- In a survey of the top 50 basic organic and inorganic chemicals made in the United States, sodium sulphate ranked 47th in terms of quantity produced.
Resources of Sodium Sulphate
- Sodium is the sixth most abundant element in the Earth’s crust. Sodium sulphate-bearing mineral deposits are geologically young, mainly of the post-glacial age.
- Sodium sulphate is widespread in occurrence and is a common component of seawater and many saline or alkaline lakes.
- Economic reserves of natural sodium sulphate are estimated at 3.3 billion tons worldwide.
- With world production of natural sodium sulphate averaging about 2.6 million tons per year, supplies are sufficient to meet anticipated demand for several centuries.
- The quantity of synthetic sodium sulphate is dependent on the longevity of the manufacturing firms recovering by-product sulphate.
- Surface depressions or lakes that have no outlets and are fed by spring waters flowing over volcanic rocks containing sulphide minerals often yield soluble sulphide salts that are oxidised by contact with the air to produce sulphates.
Uses of Sodium Sulphate
- Sodium sulphate is used to dry organic liquids.
- As a filler in powdered home laundry detergents.
- As a fining agent which removes small air bubbles from molten glass.
- Glauber’s salt, the decahydrate, was used as a laxative which removes certain drugs such as acetaminophen from the body.
- For defrosting windows, in carpet fresheners, starch manufactured, as an additive to cattle feed.
- In the manufacture of detergents and in the Kraft process of paper pulping.
Frequently Asked Questions
What happens when sodium sulphate is reacted with barium chloride?
Sodium sulphate reacts with barium chloride in a double displacement reaction to form barium sulphate and sodium chloride. The chemical equation for this reaction is given by:
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
This reaction occurs because sodium sulphate is an electrostatically bonded ionic sulphate.
Write a short note on the solubility of sodium sulphate in water
At a temperature of 0 degrees Celsius, the solubility of anhydrous sodium sulphate in water is 47.6 grams per litre. When heated to 20 degrees Celsius, its solubility sharply increases to 139 grams per litre. Finally, at a temperature of 100 degrees Celsius, the solubility of sodium sulphate in water corresponds to 427 grams per litre.
How can sodium sulphate be prepared?
Sodium sulphate can be prepared via the Mannheim process, which is given by the following reaction:
H2SO4 + 2NaCl → Na2SO4 + 2HCl
It can also be prepared via the Hargreaves process, as shown below:
4NaCl + 2H2O + 2SO2 + O2 → 2Na2SO4 + 4HCl
Give two uses of Sodium Sulphate
- Sodium sulfate is used to dry organic liquids.
- As a filler in powdered home laundry detergents.

Glauber salts, 3 to 7 g per day in warm water, said to reduce arthritis, or the occurrence of the related pain.
Please comment
Glauber’s salt, the decahydrate was used as a laxative which removes certain drugs such as acetaminophen from the body. It also reduces blood platelet adhesiveness and thus can decrease diabetes cardiovascular complications.