Stephen Reaction Mechanism Begins with the Addition of Gaseous Hydrogen Chloride to the Given Nitrile. The named reaction – Stephen aldehyde synthesis, was named after its inventor Henry Stephen. The reaction describes the preparation of aldehydes from nitriles with the help of tin(II) chloride and hydrochloric acid and the quenching of the resulting iminium salt with water. Ammonium chloride is another useful by-product produced by this reaction.
Table of Contents
Stephen Aldehyde Synthesis
An example where the Stephen reaction is used is when acetaldehyde is produced from methyl cyanide as shown below.
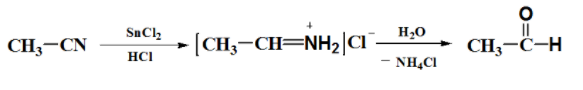
As shown above, there is an imine intermediate formed when the nitrile undergoes reduction with stannous chloride and hydrogen chloride gas (in ethyl acetate solvent). This imine intermediate is subjected to hydrolysis to yield the corresponding aldehyde.
Stephen Reaction Mechanism
Step 1
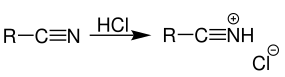
Gaseous hydrogen chloride is added to the given nitrile, which reacts to its corresponding salt as shown below.
Step 2
A single electron transfer from the tin(II) chloride reduces this salt as shown below
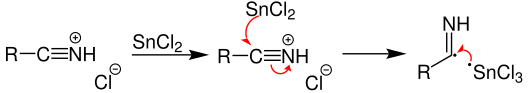
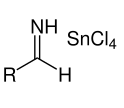
With further reaction with HCl, the following salt is formed.
Step 3
The salt obtained in step 2 precipitates shortly after as aldimine tin chloride as shown below:
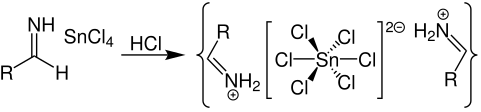
Step 4
The hydrolysis of aldimine tin chloride yields an amide. The required aldehyde is formed from this amide as shown:
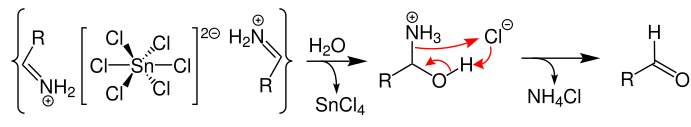
Thus, the required aldehyde is achieved from the nitrile provided. It is important to note that the reaction is more efficient when aromatic nitriles are used instead of using aliphatic nitriles. The reaction is an organic redox reaction. Substitutes that can improve the electron density will improve the formation of the aldimine tin chloride. Electron withdrawing substituents also promote the formation of amide chloride. The quenching of the aldimine tin chloride with water yields the amide, from which the aldehyde is formed. Click here to learn about more named reactions.

Comments