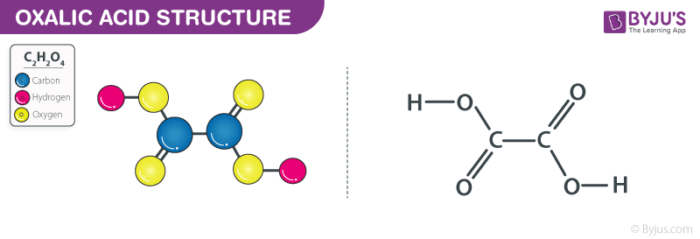
Oxalic acid which is also called ethanedioic acid is a type of dicarboxylic acid is a compound found naturally in many vegetables and plants. When it is in a solid state, it is odourless, colourless but takes the form of a white crystal substance after purification.
Oxalic acid is also produced in the human body by the metabolism of ascorbic acid or glyoxylic acid. It is removed from the body through urine. However, industrially this acid is used as a general reducing agent. It is further corrosive in nature and has a higher toxicity level than other substances. It is important to dispose this chemical properly after use.
List of Uses of Oxalic Acid
Talking about some uses of oxalic acid, this chemical is basically an essential household product that is used mainly as a cleaner for various things. In any case, we will look at some applications of this acid below.
- Cleaning Agent
- Industrial Uses
- Medicinal uses
- Other Uses
1. Cleaning Agent
Oxalic acid as mentioned above is mainly used for the toughest cleaning duties. The acid features bleach-like qualities and can be used for things like removing rust and stains on objects and metals. This acid is found in some quantity in several cleaning products, detergents and bleaches. Oxalic acid is also ideal for polishing practically any stone and treating old wood.
2. Industrial Uses
In industries, this chemical is applied mostly for mineral processing mechanisms. Additionally, oxalic acid can be used to sterilize equipment and people in the textile space use it for bleaching clothes.
3. Medicinal Uses of Oxalic Acid
In the medical field, companies make use of acid to purify certain chemicals or dilute them. However, there are very little data on the health benefits of this acid. Oxalic acid in organic and raw form is non-lethal. But sometimes it can have harmful effects on the body as well.
4. Other Uses
So, in addition to using the chemical for bleaching, and removing rust and stain, oxalic acid is mostly used as a reducing element in developing photographic film. It is also used in wastewater treatment where this acid removes calcium deposits effectively from water.
Also, Read: Preparation of Standard Solution of Oxalic Acid
Frequently Asked Questions – FAQs
Is oxalic acid good for the skin?
Extreme skin inflammation can be caused by oxalic acid. If ingested by the skin, the crystals/solution are toxic. Rare chemical burns from oxalic acid may occur and can cause hypocalcemia. Gangrene has been observed in the people who handled oxalic acid without gloves.
Does vinegar have oxalic acid?
As a matter of course, oxalic acid is a chemical element. Inherently, oxalic acid is a potent acid: it is approximately 3,000 times stronger than acetic acid, which in ordinary vinegar is the chemical term for the acid (usually marketed as about 5 percent acetic acid solution).
Why is oxalic acid used as the primary standard?
A primary standard is any material that can be exactly weighed out in pure form, such as oxalic acid, so that the number of moles present can be estimated reliably by the measured weight and the known molar mass. Main standards need not necessarily be the standard solutions used in the acid-base titration.
What can I use instead of oxalic acid?
Hydrochloric acid acts similarly to sulphuric acid as a substitute for oxalic acid. Until the steel or other metal is further worked into more economically usable forms, hydrochloric acid eliminates rust and iron oxide in metals (a method also referred to as pickling).
How do you neutralize oxalic acid?
To neutralize the symptoms of oxalic (and hydrochloric) acids, after soaking, scrub for a minute or more in running water; immerse in baking soda solution before drying. To be successful, this can take 24 hours or more.

Comments