Chromium III nitrate describes a group of inorganic compounds that are comprised of chromium, nitrates with varying amount of water. Commercially, it is of less importance, finding its application in the dyeing industry. They are used in academic laboratories for the synthesis of chromium coordination complexes. Here, in the article, learn about the Chromium III nitrate formula, along with its chemical structure and properties.
Chromium III Nitrate Properties
| Properties of Chromium III Nitrate | |
| Name | Chromium III Nitrate |
| Other Names | Nitric acid, Chromium (3+) salt |
| Appearance | Blue-violet crystals |
| Chemical Formula | Cr(NO3)3 (anhydrous) [Cr(H2O)6](NO3)3. 3H2O (nonahydrate) |
| Melting Point | 60.06 °C (nonahydrate) |
| Boiling Point | > 100 °C |
| Density | 1.85 g/cm3 |
| Molar Mass | 238.011 g/mol (anhydrous)
400.21 g/mol (nonahydrate) |
| Solubility in Water | Soluble |
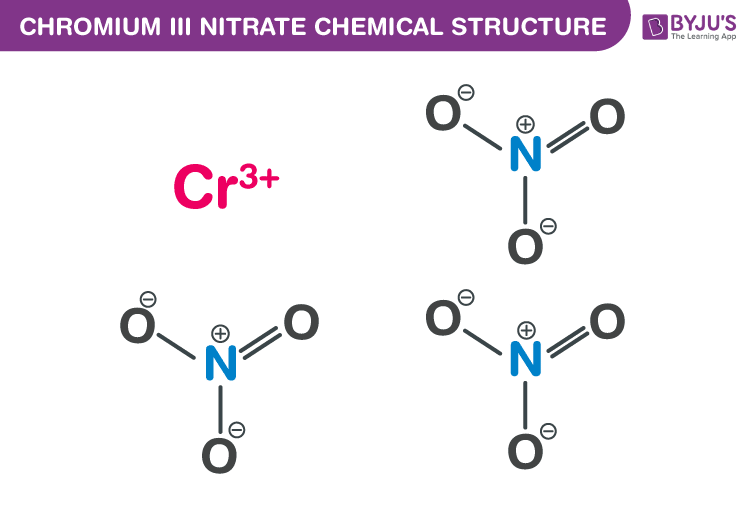
Chromium III Nitrate Chemical Structure
Chromium III Nitrate Uses
- Used in the production of alkali-metal free catalyst
- Used in the process of pickling
To learn more about such chemistry topics register to BYJU’S now!
Comments