Fluorine is a chemical element with an atomic number 9 and is denoted by F. While Fluorine gas is an elemental form of the element fluorine at standard temperature and pressure. The fluorine gas formula is F2. Fluorine gas doesn’t exist freely in nature due to its high reactivity. In this article, let us learn more about the fluorine gas formula, its chemical structure, properties and uses.
Fluorine Gas Properties
| Properties of Fluorine Gas | |
| Name | Fluorine Gas |
| Appearance | Pale yellow gas |
| Chemical Formula | F2 |
| Melting Point | −219.67°C |
| Boiling Point | −188.11 °C |
| Density | 1.696 g/L |
| Molar Weight | 37.996806 g/mol |
| Distinguishing Factor | Pale yellow gas with a pungent odour |
Fluorine Gas Chemical Structure
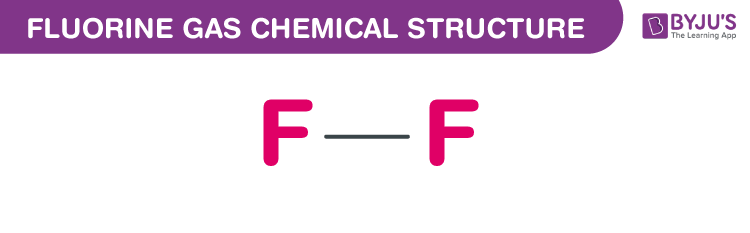
Fluorine Gas Uses
- Used in the plasma etching process of semiconductor manufacturing
- Used in the flat panel display production
- Used in the microelectromechanical systems fabrication
Related Videos
To learn more about such chemistry topics register to BYJU’S now!
Comments