Iron(II) sulfate formula, also known as Ferrous Sulfate formula or Green vitriol formula is discussed in this article. It is a compound of sulfate and iron in where the ratio of iron to sulfate ion is 1:1. Some hydrates that occur naturally are monohydrate and tetrahydrate. The molecular or chemical formula of Iron (II) sulfate is FeSO4.
Iron(II) sulfate is a crystalline solid either greenish or yellow-brown. It is dangerous to the environment and quick measures should be taken to stop it. It acts as a reducing agent. It is an iron molecule entity. It is widely used in water sewage treatment and as an ingredient in fertilizers.
Iron(II) sulfate Formula Structure
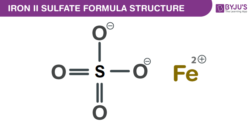
Properties Of Iron(II) sulfate Formula
| Chemical formula | FeSO4 |
| Molecular weight | 151.91 g/mol (anhydrous)
169.93 g/mol (monohydrate 241.99 g/mol (pentahydrate) 260.00 g/mol (hexahydrate) 278.02 g/mol (heptahydrate) |
| Density | 3.65 g/cm3 (anhydrous)
3 g/cm3 (monohydrate) 2.15 g/cm3 (pentahydrate) 1.934 g/cm3 (hexahydrate) 1.895 g/cm3 (heptahydrate) |
| Appears as | White crystal (anhydrous)
White-yellow crystals (monohydrate) Blue-green crystals (heptahydrate) |
| Melting point | 680 °C (anhydrous)
300 °C (monohydrate) |
Safety Measures
- Iron (II) sulfate can cause irritation in the respiratory tract.
- In its pure or concentrated form, is extremely dangerous which can lead to diarrhoea.
- It can damage blood vessels and kill a person.
To learn more about Iron(II) sulfate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Green vitriol for free.
Comments