Conformational isomerism is a form of stereoisomerism where interconversions of isomers are possible by rotations referring to single bonds. These isomers are termed as conformational isomers. Rotational energy acts as a barrier in the case of single-bond rotation. It has to be overcome to interconvert one conformer to another. The energy barrier must be small for Conformational Isomerism to occur. There are several types of conformational isomers. For example, Ethane and Butane.
Conformations of Ethane
Ethane is an organic chemical compound. It is a colourless and odourless gas at a standard temperature. Ethane molecule consists of seven sigma bonds. There will be a change in the shape of the molecule when there is a rotation of about six carbon-hydrogen bonds. But many possible differences occur when there is a rotation about the carbon-carbon bond.
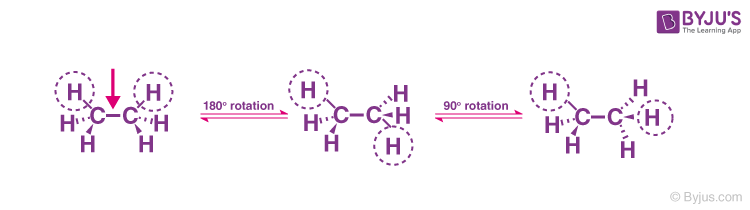
Suppose we rotate the CH3 group clockwise at an angle of 60 degrees; there would be a possibility that the hydrogen present at the front carbon is close to the hydrogen present at the back carbon. This is called eclipsed conformation.
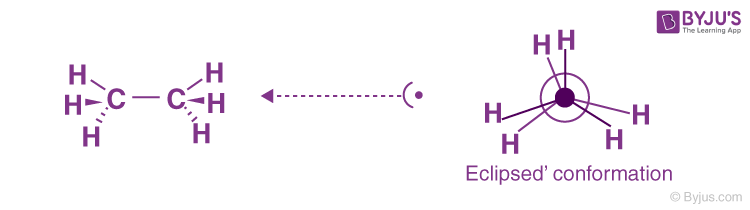
Eclipse conformation is one of the highest conformations. Another clockwise rotation at an angle of 60 degrees would lead to a second eclipsed conformation. The solid line in the above figure represents the 6 carbon-hydrogen bonds that are extended at an angle of 120 degrees from 2 carbons.
Uses of Ethane: It is widely used in the production of ethylene. It is mainly done through steam cracking. It acts as a ripening agent for food. It is a primary ingredient in mustard gas.
Conformations of Butane
Butane is an organic compound which consists of an alkane with 4 carbon atoms. It may refer to a mixture of 3 isomers. At atmospheric pressure, butane is a gas. They are liquefied gas that is highly flammable.
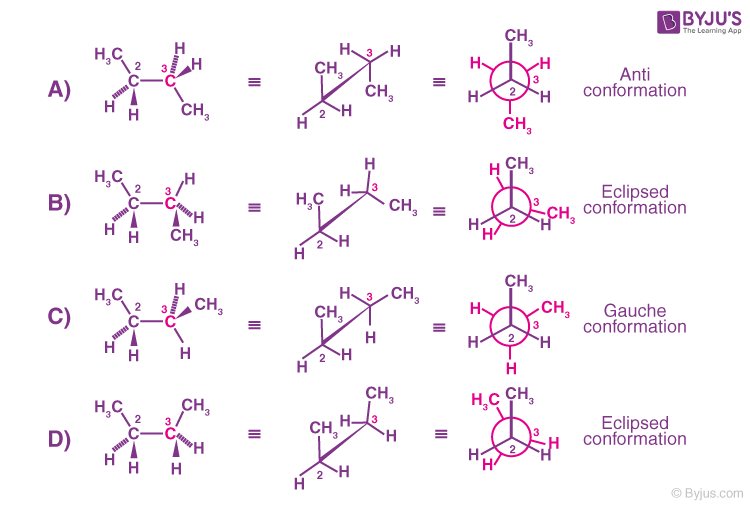
Compared to ethane, hydrogen butane consists of a complex set of conformations that is related to the constitution. The below figure represents the 4 conformations of butane.
The above diagram explains the rotation of the C2-C3 bond due to the change in potential energy.
Uses of Butane: Pure form of butane can be used as a refrigerant. It is used in Butane Torches. It is widely used in gasoline blending. Butane Cartridges are used in powered cordless hair irons.
Factors Affecting the Stability of Conformations
Conformations of Substituted Cyclohexane

Comments