Bremsstrahlung (Braking Radiation) transitions create the phenomenon of continuous x-rays whereas regular characteristic x-rays are created by inner shell transitions. Bremsstrahlung (sudden deflection or slowing down of charged particles) mechanism can be viewed when a target made of metal suffers electron bombardment. The atoms of the metal target scatter the electrons, whose change in acceleration causes a phenomenon of radiation in them.
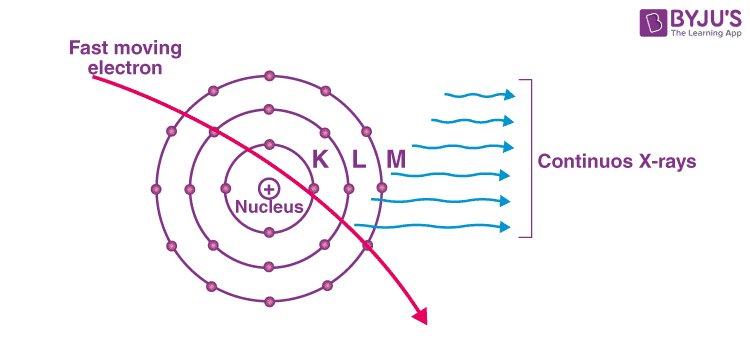
What Are Continuous X – Rays?
Just like the phenomenon of visible light, continuous X-ray spectra also contains photons ranging through a lot of wavelengths. As we all are well aware by now, the production of X-rays happens when the target which is made up of an element with a high atomic number is hit by electrons travelling at a high velocity. Most of the energy applied is wasted by being converted into heat energy in the target material’s system. X-rays that have continuously unstable wavelengths are produced due to the loss of energy that the few electrons who were moving fast enough (and penetrated to the interior sections of the atoms of the material being targeted) suffer. The attractive pulling forces applied by the nucleus of the target element causes a deceleration of these fast-moving electrons, in turn, decreasing their energy continuously. A varying frequency of X-rays is emitted continuously due to the retardation of the speed of electrons. The X – rays consist of a continuous range of frequencies up to a maximum frequency fmax or minimum wavelength λmin. This is called continuous X – rays. The minimum wavelength depends on the anode voltage. If V is the potential difference between the anode and the cathode.
eV = hfmax = hc / λmin
The minimum wavelength of the given radiation is,
λmin = hc /eV
Where the Planck’s constant is h, the velocity of light is c and the charge of the electron is e. Substituting the known values in the above equation, we get
λmin = 12400/V A0
For these specific operating voltages, the minimum wavelength is the same for all metals.
Atoms and X-Rays – Important Topics

Atoms and X-Rays – Important Questions

Frequently Asked Questions on Continuous X-rays
What is the distinction between a continuous X-ray and a characteristic X-ray?
When free moving electrons interact electromagnetically with nuclei, continuous X-rays are produced, whereas characteristic X-rays are produced during the electron transition processes that occur when an inner shell electron is liberated from an atom.
How are continuous X-rays produced?
Continuous x-rays are produced when high-velocity electrons collide with a high-atomic-number target atom.
What is the purpose of an X-ray?
Internal tissues, bones, and organs are imaged on film or digital media using invisible electromagnetic radiation beams.

Comments